Protein folding modulates the chemical reactivity of a Gram-positive adhesin
- PMID: 33257887
- PMCID: PMC7858226
- DOI: 10.1038/s41557-020-00586-x
Protein folding modulates the chemical reactivity of a Gram-positive adhesin
Abstract
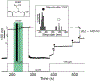
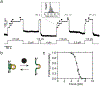
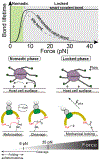
Gram-positive bacteria colonize mucosal tissues, withstanding large mechanical perturbations such as coughing, which generate shear forces that exceed the ability of non-covalent bonds to remain attached. To overcome these challenges, the pathogen Streptococcus pyogenes utilizes the protein Cpa, a pilus tip-end adhesin equipped with a Cys-Gln thioester bond. The reactivity of this bond towards host surface ligands enables covalent anchoring; however, colonization also requires cell migration and spreading over surfaces. The molecular mechanisms underlying these seemingly incompatible requirements remain unknown. Here we demonstrate a magnetic tweezers force spectroscopy assay that resolves the dynamics of the Cpa thioester bond under force. When folded at forces <6 pN, the Cpa thioester bond reacts reversibly with amine ligands, which are common in inflammation sites; however, mechanical unfolding and exposure to forces >6 pN block thioester reformation. We hypothesize that this folding-coupled reactivity switch (termed a smart covalent bond) could allow the adhesin to undergo binding and unbinding to surface ligands under low force and remain covalently attached under mechanical stress.
Figures
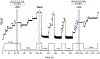
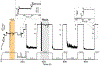




References
-
- Hall-Stoodley L, Costerton JW & Stoodley P Bacterial biofilms: from the Natural environment to infectious diseases. Nat. Rev. Microbiol 2, 95–108 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

