Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome?
- PMID: 33257910
- PMCID: PMC7491255
- DOI: 10.1183/23120541.00542-2020
Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome?
Abstract
Background: Many patients with COVID-19 did not require hospitalisation, nor underwent COVID-19 testing. There is anecdotal evidence that patients with "mild" COVID-19 may complain about persistent symptoms, even weeks after the infection. This suggests that symptoms during the infection may not resolve spontaneously. The objective of this study was to assess whether multiple relevant symptoms recover following the onset of symptoms in hospitalised and nonhospitalised patients with COVID-19.
Methods: A total of 2113 members of two Facebook groups for coronavirus patients with persistent complaints in the Netherlands and Belgium, and from a panel of people who registered on a website of the Lung Foundation Netherlands, were assessed for demographics, pre-existing comorbidities, health status, date of symptoms onset, COVID-19 diagnosis, healthcare utilisation, and the presence of 29 symptoms at the time of the onset of symptoms (retrospectively) and at follow-up (mean±sd 79±17 days after symptoms onset).
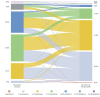
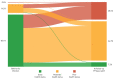
Results: Overall, 112 hospitalised patients and 2001 nonhospitalised patients (confirmed COVID-19, n=345; symptom-based COVID-19, n=882; and suspected COVID-19, n=774) were analysed. The median number of symptoms during the infection reduced significantly over time (median (interquartile range) 14 (11-17) versus 6 (4-9); p<0.001). Fatigue and dyspnoea were the most prevalent symptoms during the infection and at follow-up (fatigue: 95% versus 87%; dyspnoea: 90% versus 71%).
Conclusion: In previously hospitalised and nonhospitalised patients with confirmed or suspected COVID-19, multiple symptoms are present about 3 months after symptoms onset. This suggests the presence of a "post-COVID-19 syndrome" and highlights the unmet healthcare needs in a subgroup of patients with "mild" or "severe" COVID-19.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: Y.M.J. Goërtz has nothing to disclose. Conflict of interest: M. Van Herck has nothing to disclose. Conflict of interest: J.M. Delbressine has nothing to disclose. Conflict of interest: A.W. Vaes has nothing to disclose. Conflict of interest: R. Meys has nothing to disclose. Conflict of interest: F.V.C. Machado has nothing to disclose. Conflict of interest: S. Houben-Wilke has nothing to disclose. Conflict of interest: C. Burtin has nothing to disclose. Conflict of interest: R. Posthuma has nothing to disclose. Conflict of interest: F.M.E. Franssen reports personal fees from GlaxoSmithKline, Chiesi and Boehringer Inghelheim, grants and personal fees from AstraZeneca and Novartis, and personal fees from TEVA, outside the submitted work. Conflict of interest: N. van Loon has nothing to disclose. Conflict of interest: B. Hajian has nothing to disclose. Conflict of interest: Y. Spies has nothing to disclose. Conflict of interest: H. Vijlbrief has nothing to disclose. Conflict of interest: A.J. van ’t Hul has nothing to disclose. Conflict of interest: D.J.A. Janssen reports speaker fees from Novartis, Boehringer Ingelheim and AstraZeneca, outside the submitted work. Conflict of interest: M.A. Spruit reports grants from Lung Foundation Netherlands and Stichting Astma Bestrijding, and grants and personal fees from Boehringer Ingelheim and AstraZeneca, outside the submitted work.
Figures

References
-
- Johns Hopkins University & Medicine. Coronavirus Resource Center https://coronavirus.jhu.edu/ Date last updated: 8 July, 2020. Date last accessed: 9 July, 2020.
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
