Direct oral anticoagulant use and risk of severe COVID-19
- PMID: 33258156
- PMCID: PMC7753564
- DOI: 10.1111/joim.13205
Direct oral anticoagulant use and risk of severe COVID-19
Abstract
Background: Hypercoagulability and thromboembolism are prominent features of severe COVID-19, and ongoing anticoagulant use might be protective.
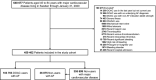
Methods: We conducted a nationwide register-based cohort study in Sweden, February through May, 2020, to assess whether ongoing direct oral anticoagulant (DOAC) use was associated with reduced risk of hospital admission for laboratory-confirmed COVID-19, or a composite of intensive care unit (ICU) admission or death due to laboratory-confirmed COVID-19.
Results: DOAC use (n = 103 703) was not associated with reduced risk of hospital admission for COVID-19 (adjusted hazard ratio [aHR] [95% confidence interval] 1.00 [0.75-1.33] vs. nonuse atrial fibrillation comparator [n = 36 875]; and aHR 0.94 [0.80-1.10] vs. nonuse cardiovascular disease comparator [n = 355 699]), or ICU admission or death due to COVID-19 (aHRs 0.76 [0.51-1.12], and 0.90 [0.71-1.15], respectively).
Conclusion: Ongoing DOAC use was not associated with reduced risk of severe COVID-19, indicating that prognosis would not be modified by early outpatient DOAC initiation.
Keywords: COVID-19; SARS-CoV-2; anticoagulants; atrial fibrillation; direct-acting oral anticoagulants.
© 2020 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
Dr. Ludvigsson coordinates a study on behalf of the Swedish Inflammatory Bowel Disease Register (SWIBREG). This study has received funding from Janssen Corporation. The remaining authors declare no competing financial interests.
Figures
Comment in
-
The pathogenesis of microthrombi in COVID-19 cannot be controlled by DOAC: NETosis should be the target.J Intern Med. 2021 Mar;289(3):420-421. doi: 10.1111/joim.13228. Epub 2021 Jan 10. J Intern Med. 2021. PMID: 33423337 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous