The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo
- PMID: 33258480
- PMCID: PMC7986223
- DOI: 10.1113/JP280652
The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo
Abstract
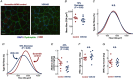
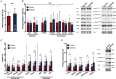
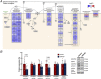
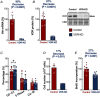
Key points: Reduced vitamin D receptor (VDR) expression prompts skeletal muscle atrophy. Atrophy occurs through catabolic processes, namely the induction of autophagy, while anabolism remains unchanged. In response to VDR-knockdown mitochondrial function and related gene-set expression is impaired. In vitro VDR knockdown induces myogenic dysregulation occurring through impaired differentiation. These results highlight the autonomous role the VDR has within skeletal muscle mass regulation.
Abstract: Vitamin D deficiency is estimated to affect ∼40% of the world's population and has been associated with impaired muscle maintenance. Vitamin D exerts its actions through the vitamin D receptor (VDR), the expression of which was recently confirmed in skeletal muscle, and its down-regulation is linked to reduced muscle mass and functional decline. To identify potential mechanisms underlying muscle atrophy, we studied the impact of VDR knockdown (KD) on mature skeletal muscle in vivo, and myogenic regulation in vitro in C2C12 cells. Male Wistar rats underwent in vivo electrotransfer (IVE) to knock down the VDR in hind-limb tibialis anterior (TA) muscle for 10 days. Comprehensive metabolic and physiological analysis was undertaken to define the influence loss of the VDR on muscle fibre composition, protein synthesis, anabolic and catabolic signalling, mitochondrial phenotype and gene expression. Finally, in vitro lentiviral transfection was used to induce sustained VDR-KD in C2C12 cells to analyse myogenic regulation. Muscle VDR-KD elicited atrophy through a reduction in total protein content, resulting in lower myofibre area. Activation of autophagic processes was observed, with no effect upon muscle protein synthesis or anabolic signalling. Furthermore, RNA-sequencing analysis identified systematic down-regulation of multiple mitochondrial respiration-related protein and genesets. Finally, in vitro VDR-knockdown impaired myogenesis (cell cycling, differentiation and myotube formation). Together, these data indicate a fundamental regulatory role of the VDR in the regulation of myogenesis and muscle mass, whereby it acts to maintain muscle mitochondrial function and limit autophagy.
Keywords: atrophy; metabolism; skeletal muscle; vitamin D.
© 2020 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures



Comment in
-
Novel insights into the autonomous role played by vitamin D receptor in the regulation of skeletal muscle mass.J Physiol. 2021 Apr;599(7):1955-1956. doi: 10.1113/JP281211. Epub 2021 Feb 2. J Physiol. 2021. PMID: 33476041 No abstract available.
References
-
- Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R & Demay MB (1999). Rescue of the skeletal phenotype of vitamin D receptor‐ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology, 140, 4982–4987. - PubMed
-
- Andrews S. (2010). FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc
-
- Arnold DL, Matthews PM & Radda GK (1984). Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med 1, 307–315. - PubMed
-
- ( Arthur W. Ham MDL1934). Hypervitaminosis D rickets: the action of vitamin D. Br J Exp Pathol 15, 228. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2065028/ [Accessed 20 April 2016].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

