TREM-1 orchestrates angiotensin II-induced monocyte trafficking and promotes experimental abdominal aortic aneurysm
- PMID: 33258804
- PMCID: PMC7810476
- DOI: 10.1172/JCI142468
TREM-1 orchestrates angiotensin II-induced monocyte trafficking and promotes experimental abdominal aortic aneurysm
Abstract
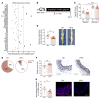
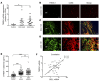
The triggering receptor expressed on myeloid cells 1 (TREM-1) drives inflammatory responses in several cardiovascular diseases but its role in abdominal aortic aneurysm (AAA) remains unknown. Our objective was to explore the role of TREM-1 in a mouse model of angiotensin II-induced (AngII-induced) AAA. TREM-1 expression was detected in mouse aortic aneurysm and colocalized with macrophages. Trem1 gene deletion (Apoe-/-Trem1-/-), as well as TREM-1 pharmacological blockade with LR-12 peptide, limited both AAA development and severity. Trem1 gene deletion attenuated the inflammatory response in the aorta, with a reduction of Il1b, Tnfa, Mmp2, and Mmp9 mRNA expression, and led to a decreased macrophage content due to a reduction of Ly6Chi classical monocyte trafficking. Conversely, antibody-mediated TREM-1 stimulation exacerbated Ly6Chi monocyte aorta infiltration after AngII infusion through CD62L upregulation and promoted proinflammatory signature in the aorta, resulting in worsening AAA severity. AngII infusion stimulated TREM-1 expression and activation on Ly6Chi monocytes through AngII receptor type I (AT1R). In human AAA, TREM-1 was detected and TREM1 mRNA expression correlated with SELL mRNA expression. Finally, circulating levels of sTREM-1 were increased in patients with AAA when compared with patients without AAA. In conclusion, TREM-1 is involved in AAA pathophysiology and may represent a promising therapeutic target in humans.
Keywords: Cell migration/adhesion; Inflammation; Innate immunity; Monocytes; Vascular Biology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

