Trends in Dispensing of Zolpidem and Low-Dose Trazodone Among Commercially Insured Adults in the United States, 2011-2018
- PMID: 33258882
- PMCID: PMC7709087
- DOI: 10.1001/jama.2020.19224
Trends in Dispensing of Zolpidem and Low-Dose Trazodone Among Commercially Insured Adults in the United States, 2011-2018
Abstract
This study uses IBM MarketScan database data to describe trends in zolpidem and low-dose trazodone dispensing among adults with employer-sponsored insurance or Medicare supplemental plans between 2011 and 2018, before and after a 2017 clinical practice guideline discouraged trazodone use for insomnia.
Conflict of interest statement
Figures
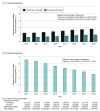
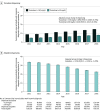
References
-
- US Food and Drug Administration FDA drug safety communication: FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR. Published May 14, 2013. Accessed June 30, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-c...

