Assessment of Cancer Therapy Evaluation Program Advocacy and Inclusion Rates of People Living With HIV in Anti-PD1/PDL1 Clinical Trials
- PMID: 33258905
- PMCID: PMC7709086
- DOI: 10.1001/jamanetworkopen.2020.27110
Assessment of Cancer Therapy Evaluation Program Advocacy and Inclusion Rates of People Living With HIV in Anti-PD1/PDL1 Clinical Trials
Abstract
Importance: Anti-programmed death 1 and anti-programmed death ligand 1 (anti-PD1/PDL1) immune checkpoint blockade (ICB) constitutes the therapeutic backbone for multiple malignant neoplasms. People living with HIV (PLWH) have routinely been excluded from ICB clinical trials, thus inhibiting broad implementation of ICB to PLWH with cancer.
Objective: To evaluate trends in the inclusion of PLWH in ICB cancer clinical trials that have occurred in association with ongoing efforts by the Cancer Therapy Evaluation Program (CTEP), National Cancer Institute, to promote inclusion of PLWH.
Design, setting, and participants: This quality improvement study of ICB letters of intent (LOIs) included anti-PD1/PDL1 agents (nivolumab, pembrolizumab, atezolizumab, and durvalumab) submitted to CTEP that proceeded to approved protocols between January 2014 to May 2019. The setting was ICB clinical trial development and inclusion of underrepresented populations, specifically PLWH. All 97 submitted cancer clinical trial LOIs that included the aforementioned ICB agents were eligible for inclusion. Ten proposals were excluded, of which 3 were designed specifically for PLWH and 7 were LOIs that did not advance to approved protocols within the study period. Statistical analysis was performed from April to September 2020.
Exposures: CTEP advocacy included the requirement for justification of exclusion of PLWH and formal discussion of inclusion criteria during conference calls between CTEP and trial investigators.
Main outcomes and measures: The frequency of inclusion of PLWH in initially submitted LOIs was compared with final approved protocols using descriptive statistics. The probability of inclusion of PLWH in submitted LOIs and approved protocols over time was assessed using logistic regression.
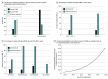
Results: Eighty-seven studies were included, of which 68 (78%) were pilot, phase 1, phase 1/2, or phase 2 studies and 19 (22%) were phase 2/3 or phase 3 studies. Thirty-nine studies (45%) included nivolumab, 23 (26%) included pembrolizumab, 19 (22%) included atezolizumab, and 6 (7%) included durvalumab. At initial LOI stage, 14 of 87 (16%) included PLWH. Following CTEP advocacy efforts, 61 of 87 protocols (70%) included PLWH. Of 36 LOIs to initially exclude PLWH, 24 (67%) included PLWH in final protocols. Among the 25 protocols to exclude PLWH, 21 (84%) were earlier phase studies (pilot to phase 2) and 4 (16%) were later phase studies (phase 2/3 to phase 3). Only 13 of 25 protocols (52%) provided justification for exclusion of PLWH, with safety being the most frequently cited concern (9 of 13 studies). The inclusion of PLWH on submitted LOIs increased over time (odds ratio, 3.38; 95% CI, 1.14-3.91), whereas inclusion on final protocols did not increase over time (odds ratio, 1.80; 95% CI, 0.81-1.59).
Conclusions and relevance: This study identified encouraging trends in the inclusion of PLWH in anti-PD1/PDL1 cancer trials that occurred in the period following the initiation of CTEP advocacy. Work is needed to examine what impact this will have on enrollment of PLWH in such trials. Similar advocacy may help to promote inclusion of other underrepresented populations in cancer clinical trials, including those with organ dysfunction and chronic infections.
Conflict of interest statement
Figures
Similar articles
-
Anti-PD1/PDL1 induced psoriasis.Curr Probl Cancer. 2017 Nov-Dec;41(6):407-412. doi: 10.1016/j.currproblcancer.2017.10.003. Epub 2017 Oct 18. Curr Probl Cancer. 2017. PMID: 29096940
-
Exclusion of patients living with HIV from cancer immune checkpoint inhibitor trials.Sci Rep. 2021 Mar 23;11(1):6637. doi: 10.1038/s41598-021-86081-w. Sci Rep. 2021. PMID: 33758321 Free PMC article.
-
Dual Checkpoint Inhibition with Ipilimumab plus Nivolumab After Progression on Sequential PD-1/PDL-1 Inhibitors Pembrolizumab and Atezolizumab in a Patient with Lynch Syndrome, Metastatic Colon, and Localized Urothelial Cancer.Oncologist. 2019 Nov;24(11):1416-1419. doi: 10.1634/theoncologist.2018-0686. Epub 2019 Aug 23. Oncologist. 2019. PMID: 31444293 Free PMC article.
-
Immunotherapy revolutionises non-small-cell lung cancer therapy: Results, perspectives and new challenges.Eur J Cancer. 2017 Jun;78:16-23. doi: 10.1016/j.ejca.2016.12.041. Epub 2017 Apr 11. Eur J Cancer. 2017. PMID: 28407528 Review.
-
Anti-PD-1 and Anti-PD-L1 Monoclonal Antibodies in People Living with HIV and Cancer.Curr HIV/AIDS Rep. 2020 Oct;17(5):547-556. doi: 10.1007/s11904-020-00525-y. Curr HIV/AIDS Rep. 2020. PMID: 32827111 Free PMC article. Review.
Cited by
-
Immunotherapy and Gene Therapy for Oncoviruses Infections: A Review.Viruses. 2021 May 2;13(5):822. doi: 10.3390/v13050822. Viruses. 2021. PMID: 34063186 Free PMC article. Review.
-
Immune Checkpoint Inhibitors in People Living with HIV/AIDS: Facts and Controversies.Cells. 2021 Aug 27;10(9):2227. doi: 10.3390/cells10092227. Cells. 2021. PMID: 34571876 Free PMC article. Review.
-
The Challenging Scenario of Cancer Treatment for People with HIV: Clinical Experience with Immune Checkpoint Inhibitors.Curr Oncol. 2025 Mar 13;32(3):164. doi: 10.3390/curroncol32030164. Curr Oncol. 2025. PMID: 40136368 Free PMC article.
-
A clinical overview of people living with HIV and genitourinary cancer care.Nat Rev Urol. 2024 Jun;21(6):373-383. doi: 10.1038/s41585-023-00846-8. Epub 2024 Jan 18. Nat Rev Urol. 2024. PMID: 38238527 Review.
-
Treating Cancer in People With HIV.J Clin Oncol. 2023 Jul 20;41(21):3682-3688. doi: 10.1200/JCO.23.00737. Epub 2023 Jun 2. J Clin Oncol. 2023. PMID: 37267514 Free PMC article. Review.
References
-
- Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi:10.1371/journal.pone.0081355 - DOI - PMC - PubMed
-
- Silverberg MJ, Lau B, Achenbach CJ, et al. ; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS . Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507-518. doi:10.7326/M14-2768 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

