Re-emergence of yellow fever in the neotropics - quo vadis?
- PMID: 33258924
- PMCID: PMC7733675
- DOI: 10.1042/ETLS20200187
Re-emergence of yellow fever in the neotropics - quo vadis?
Abstract
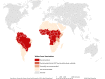
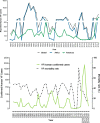
Yellow fever virus (YFV) is the etiological agent of yellow fever (YF), an acute hemorrhagic vector-borne disease with a significant impact on public health, is endemic across tropical regions in Africa and South America. The virus is maintained in two ecologically and evolutionary distinct transmission cycles: an enzootic, sylvatic cycle, where the virus circulates between arboreal Aedes species mosquitoes and non-human primates, and a human or urban cycle, between humans and anthropophilic Aedes aegypti mosquitoes. While the urban transmission cycle has been eradicated by a highly efficacious licensed vaccine, the enzootic transmission cycle is not amenable to control interventions, leading to recurrent epizootics and spillover outbreaks into human populations. The nature of YF transmission dynamics is multifactorial and encompasses a complex system of biotic, abiotic, and anthropogenic factors rendering predictions of emergence highly speculative. The recent outbreaks in Africa and Brazil clearly remind us of the significant impact YF emergence events pose on human and animal health. The magnitude of the Brazilian outbreak and spillover in densely populated areas outside the recommended vaccination coverage areas raised the specter of human - to - human transmission and re-establishment of enzootic cycles outside the Amazon basin. Herein, we review the factors that influence the re-emergence potential of YFV in the neotropics and offer insights for a constellation of coordinated approaches to better predict and control future YF emergence events.
Keywords: arbovirus; epizootics; outbreak; re-emergence; transmission cycles; yellow fever virus.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
-
- Rush A.B. (1789) An Account of the Bilious Remitting Fever, as it Appeared in Philadelphia in the Summer and Autumn of the Year 1780. Medical Inquiries and Observations, Prichard and Hall, Philadelphia
-
- Stokes A., Bauer J.H. and Hudson N.P. (1928) Experimental transmission of yellow fever to Macacus rhesus. A preliminary note. J. Amer. Med. Assoc. 90, 253 10.1001/jama.1928.02690310005002 - DOI
-
- Carrigan J.A. (1967) Yellow fever in New orleans, 1905: the last epidemic. Bull. Tulane Med. Fac. 26, 19–28 PMID: - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
