Conditional reprogramming culture conditions facilitate growth of lower-grade glioma models
- PMID: 33258947
- PMCID: PMC8099469
- DOI: 10.1093/neuonc/noaa263
Conditional reprogramming culture conditions facilitate growth of lower-grade glioma models
Abstract
Background: The conditional reprogramming cell culture method was developed to facilitate growth of senescence-prone normal and neoplastic epithelial cells, and involves co-culture with irradiated fibroblasts and the addition of a small molecule Rho kinase (ROCK) inhibitor. The aim of this study was to determine whether this approach would facilitate the culture of compact low-grade gliomas.
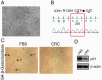
Methods: We attempted to culture 4 pilocytic astrocytomas, 2 gangliogliomas, 2 myxopapillary ependymomas, 2 anaplastic gliomas, 2 difficult-to-classify low-grade neuroepithelial tumors, a desmoplastic infantile ganglioglioma, and an anaplastic pleomorphic xanthoastrocytoma using a modified conditional reprogramming cell culture approach.
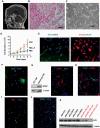
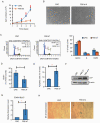
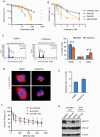
Results: Conditional reprogramming resulted in robust increases in growth for a majority of these tumors, with fibroblast conditioned media and ROCK inhibition both required. Switching cultures to standard serum containing media, or serum-free neurosphere conditions, with or without ROCK inhibition, resulted in decreased proliferation and induction of senescence markers. Rho kinase inhibition and conditioned media both promoted Akt and Erk1/2 activation. Several cultures, including one derived from a NF1-associated pilocytic astrocytoma (JHH-NF1-PA1) and one from a BRAF p.V600E mutant anaplastic pleomorphic xanthoastrocytoma (JHH-PXA1), exhibited growth sufficient for preclinical testing in vitro. In addition, JHH-NF1-PA1 cells survived and migrated in larval zebrafish orthotopic xenografts, while JHH-PXA1 formed orthotopic xenografts in mice histopathologically similar to the tumor from which it was derived.
Conclusions: These studies highlight the potential for the conditional reprogramming cell culture method to promote the growth of glial and glioneuronal tumors in vitro, in some cases enabling the establishment of long-term culture and in vivo models.
Keywords: BRAFV600E; NF1; conditional reprogramming; enescence; low-grade glioma.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma.Acta Neuropathol. 2011 Mar;121(3):397-405. doi: 10.1007/s00401-011-0802-6. Epub 2011 Jan 29. Acta Neuropathol. 2011. PMID: 21274720
-
A comprehensive analysis of infantile central nervous system tumors to improve distinctive criteria for infant-type hemispheric glioma versus desmoplastic infantile ganglioglioma/astrocytoma.Brain Pathol. 2023 Sep;33(5):e13182. doi: 10.1111/bpa.13182. Epub 2023 Jun 22. Brain Pathol. 2023. PMID: 37349135 Free PMC article.
-
Clinical-pathological study of 28 glial and mixed neuronal-glial tumors diagnosed within the first year of life.Clin Neuropathol. 2022 Jan-Feb;41(1):25-34. doi: 10.5414/NP301405. Clin Neuropathol. 2022. PMID: 34622774
-
Pathologic and molecular aspects of anaplasia in circumscribed gliomas and glioneuronal tumors.Brain Tumor Pathol. 2019 Apr;36(2):40-51. doi: 10.1007/s10014-019-00336-z. Epub 2019 Mar 11. Brain Tumor Pathol. 2019. PMID: 30859342 Free PMC article. Review.
-
Prevalence of BRAFV600 in glioma and use of BRAF Inhibitors in patients with BRAFV600 mutation-positive glioma: systematic review.Neuro Oncol. 2022 Apr 1;24(4):528-540. doi: 10.1093/neuonc/noab247. Neuro Oncol. 2022. PMID: 34718782 Free PMC article.
Cited by
-
Stem cell modeling of nervous system tumors.Dis Model Mech. 2024 Feb 1;17(2):dmm050533. doi: 10.1242/dmm.050533. Epub 2024 Feb 14. Dis Model Mech. 2024. PMID: 38353122 Free PMC article. Review.
-
Visual Deficits and Diagnostic and Therapeutic Strategies for Neurofibromatosis Type 1: Bridging Science and Patient-Centered Care.Vision (Basel). 2024 May 9;8(2):31. doi: 10.3390/vision8020031. Vision (Basel). 2024. PMID: 38804352 Free PMC article. Review.
-
MOST wanted: navigating the MAPK-OIS-SASP-tumor microenvironment axis in primary pediatric low-grade glioma and preclinical models.Childs Nerv Syst. 2024 Oct;40(10):3209-3221. doi: 10.1007/s00381-024-06463-z. Epub 2024 May 25. Childs Nerv Syst. 2024. PMID: 38789691 Free PMC article. Review.
-
Human Patient-Derived Brain Tumor Models to Recapitulate Ependymoma Tumor Vasculature.Bioengineering (Basel). 2023 Jul 15;10(7):840. doi: 10.3390/bioengineering10070840. Bioengineering (Basel). 2023. PMID: 37508868 Free PMC article.
-
Preclinical modeling of lower-grade gliomas.Front Oncol. 2023 Mar 27;13:1139383. doi: 10.3389/fonc.2023.1139383. eCollection 2023. Front Oncol. 2023. PMID: 37051530 Free PMC article. Review.
References
-
- Kogiso M, Qi L, Lindsay H, et al. . Xenotransplantation of pediatric low grade gliomas confirms the enrichment of BRAF V600E mutation and preservation of CDKN2A deletion in a novel orthotopic xenograft mouse model of progressive pleomorphic xanthoastrocytoma. Oncotarget. 2017;8(50):87455–87471. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

