Dosing Considerations for Antibodies Against COVID-19
- PMID: 33259037
- PMCID: PMC7705402
- DOI: 10.1007/s40268-020-00330-3
Dosing Considerations for Antibodies Against COVID-19
Abstract
At present, no cure is available for COVID-19 but vaccines, antiviral drugs, immunoglobulins, or the combination of immunoglobulins with antiviral drugs have been suggested and are in clinical trials. The purpose of this paper is to discuss the role of a pharmacokinetic and viral load analysis as a basis for adjusting immunoglobulin dosing to treat COVID-19. We reviewed the pre-clinical and clinical literature that describes the impact of a high antigen load on pharmacokinetic data following antibody treatment. Representative examples are provided to illustrate the effect of high viral and tumor loads on antibody clearance. We then highlight the implications of these factors for facilitating the development and dosing of hyperimmune anti-SARS CoV2 immunoglobulin. Both nonclinical and clinical examples indicate that high antigen loads, whether they be viral, bacterial, or tumoral in origin, result in increased clearance and decreased area under the curve and half-life of antibodies. A dosing strategy that matches the antigen load can be achieved by giving initially high doses and adjusting the frequency of dosing intervals based on pharmacokinetic parameters. We suggest that study design and dose selection for immunoglobulin products for the treatment of COVID-19 require special considerations such as viral load, antibody-virus interaction, and dosing adjustment based on the pharmacokinetics of the antibody.
Conflict of interest statement
The authors have no conflicts of interest to declare.
No financial conflicts of interest, all the data are in the public domain.
Figures
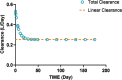
References
-
- CDC. COVID-19 pandemic planning scenarios. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. Accessed 25 Aug 2020.
-
- Mayo Clinic. Coronavirus disease 2019 (COVID-19). https://www.mayoclinic.org/diseases-conditions/coronavirus/symptoms-caus.... Accessed 15 July 2020.
-
- NIH. Potential antiviral drugs under evaluation for the treatment of COVID-19. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/. Accessed 25 Aug 2020.
-
- US FDA. FDA issues emergency use authorization for convalescent plasma as potential promising COVID-19 treatment, another achievement in administration’s fight against pandemic. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency.... Accessed 28 Aug 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

