The interaction between protein kinase A and progesterone on basal and inflammation-induced myometrial oxytocin receptor expression
- PMID: 33259490
- PMCID: PMC7707466
- DOI: 10.1371/journal.pone.0239937
The interaction between protein kinase A and progesterone on basal and inflammation-induced myometrial oxytocin receptor expression
Abstract
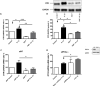
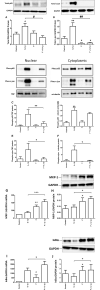
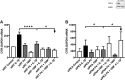
Our previous work has shown myometrial PKA activity declines in term and twin-preterm labour in association with an increase in the expression of the oxytocin receptor (OTR). Here we investigate the action of cAMP/PKA in basal conditions, with the addition of progesterone (P4) and/or IL-1β to understand how cAMP/PKA acts to maintain pregnancy and whether the combination of cAMP and P4 would be a viable therapeutic combination for the prevention of preterm labour (PTL). Further, given that we have previously found that cAMP enhances P4 action we wanted to test the hypothesis that changes in the cAMP effector system are responsible for the functional withdrawal of myometrial P4 action. Myometrial cells were grown from biopsies obtained from women at the time of elective Caesarean section before the onset of labour. The addition of forskolin, an adenylyl cyclase activator, repressed basal OTR mRNA levels at all doses and P4 only enhanced this effect at its highest dose. Forskolin repressed the IL-1β-induced increase in OTR mRNA and protein levels in a PKA-dependent fashion and repressed IL-1β-activation and nuclear transfer of NFκB and AP-1. P4 had similar effects and the combination P4 and forskolin had greater effects on OTR and NFκB than forskolin alone. While PKA knockdown had no effect on the ability of P4 to repress IL-1β-induced OTR expression it reversed the repressive effect of the combination of P4 and forskolin and resulted in a greater increase than observed with IL-1β alone. These studies suggest that cAMP acts via PKA to repress inflammation-driven OTR expression, but that when PKA activity is reduced, the combination of cAMP and P4 actually enhances the OTR response to inflammation, promoting the onset of labour and suggesting that changes in the cAMP effector system can induce a functional P4 withdrawal.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

