Cost comparison of nine-month treatment regimens with 20-month standardized care for the treatment of rifampicin-resistant/multi-drug resistant tuberculosis in Nigeria
- PMID: 33259492
- PMCID: PMC7707487
- DOI: 10.1371/journal.pone.0241065
Cost comparison of nine-month treatment regimens with 20-month standardized care for the treatment of rifampicin-resistant/multi-drug resistant tuberculosis in Nigeria
Erratum in
-
Correction: Cost comparison of nine-month treatment regimens with 20-month standardized care for the treatment of rifampicin-resistant/multi-drug resistant tuberculosis in Nigeria.PLoS One. 2021 Oct 28;16(10):e0259492. doi: 10.1371/journal.pone.0259492. eCollection 2021. PLoS One. 2021. PMID: 34710200 Free PMC article.
-
Correction: Cost comparison of nine-month treatment regimens with 20-month standardized care for the treatment of rifampicin-resistant/multi-drug resistant tuberculosis in Nigeria.PLoS One. 2025 Apr 4;20(4):e0321797. doi: 10.1371/journal.pone.0321797. eCollection 2025. PLoS One. 2025. PMID: 40184342 Free PMC article.
Abstract
Background: Globally, drug resistant tuberculosis (DR-TB) continues to be a public health threat. Nigeria, which accounts for a significant proportion of the global burden of rifampicin/multi-drug resistant-TB (RR/MDR-TB) had a funding gap of $168 million dollars for TB treatment in 2018. Since 2010, Nigeria has utilized five different models of care for RR/MDR-TB (Models A-E); Models A, B and C based on a standardized WHO-approved treatment regimen of 20-24 months, were phased out between 2015 and 2019 and replaced by Models D and E. Model D is a fully ambulatory model of 9-12 months during which a shorter treatment regimen including a second-line injectable agent is utilized. Model E is identical to Model D but has patients hospitalized for the first four months of care while Model F which is to be introduced in 2020, is a fully ambulatory, oral bedaquiline-containing shorter treatment regimen of 9-12 months. Treatment models for RR/MDR-TB of 20-24 months duration have had treatment success rates of 52-66% while shorter treatment regimens have reported success rates of 85% and above. In addition, replacing the second-line injectable agent in a shorter treatment regimen with bedaquiline has been found to further improve treatment success in patients with fluoroquinolone-susceptible RR/MDR-TB. Reliable cost data for RR/MDR-TB care are limited, specifically costs of models that utilize shorter treatment regimens and which are vital to guide Nigeria through the provision of RR/MDR-TB care at scale. We therefore conducted a cost analysis of shorter treatment regimens in use and to be used in Nigeria (Models D, E and F) and compared them to three models of longer duration utilized previously in Nigeria (Models A, B and C) to identify any changes in cost from transitioning from Models A-C to Models D-F and opportunities for cost savings.
Methods: We obtained costs for TB diagnostic and monitoring tests, in-patient and out-patient care from a previous study, inflated these costs to 2019 NGN and then converted to 2020 USD. We obtained other costs from the average of six health facilities and drug costs from the global drug facility. We modeled treatment on strict adherence to two Nigerian National guidelines for programmatic and clinical management of drug-resistant tuberculosis.
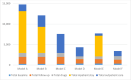
Results: We estimated that the total costs of care from the health sector perspective for Models D, E and F were $4,334, $7,705 and $3,420 respectively. This is significantly lower than the costs of Models A, B and C which were $14,781, $12, 113, $7,572 respectively.
Conclusion: Replacing Models A-C with Models D and E reduced the costs of RR/MDR-TB care in Nigeria by approximately $5,470 (48%) per patient treated and transitioning from Models D and E to Model F would result in further cost savings of $914 to $4,285 (21 to 56%) for every patient placed on Model F. If the improved outcomes of patients managed using bedaquiline-containing shorter treatment regimens in other countries can be attained in Nigeria, Model F would be the recommended model for the scale up of RR/MDR-TB care in Nigeria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization, “Global Tuberculosis Report 2019,” 2019.
-
- World Health Organization, “Guidelines for the programmatic management of Multidrug-resistant Tuberculosis.,” World Heal. Organ., p. 44, 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous