Gut mycobiomes are altered in people with type 2 Diabetes Mellitus and Diabetic Retinopathy
- PMID: 33259537
- PMCID: PMC7707496
- DOI: 10.1371/journal.pone.0243077
Gut mycobiomes are altered in people with type 2 Diabetes Mellitus and Diabetic Retinopathy
Abstract
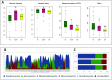
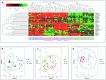
Studies have documented dysbiosis in the gut mycobiome in people with Type 2 diabetes mellitus (T2DM). However, it is not known whether dysbiosis in the gut mycobiome of T2DM patients would be reflected in people with diabetic retinopathy (DR) and if so, is the observed mycobiome dysbiosis similar in people with T2DM and DR. Gut mycobiomes were generated from healthy controls (HC), people with T2DM and people with DR through Illumina sequencing of ITS2 region. Data were analysed using QIIME and R software. Dysbiotic changes were observed in people with T2DM and DR compared to HC at the phyla and genera level. Mycobiomes of HC, T2DM and DR could be discriminated by heat map analysis, Beta diversity analysis and LEfSE analysis. Spearman correlation of fungal genera indicated more negative correlation in HC compared to T2DM and DR mycobiomes. This study demonstrates dysbiosis in the gut mycobiomes in people with T2DM and DR compared to HC. These differences were significant both at the phyla and genera level between people with T2DM and DR as well. Such studies on mycobiomes may provide new insights and directions to identification of specific fungi associated with T2DM and DR and help developing novel therapies for Diabetes Mellitus and DR.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8(2):110–20. Epub 2011/02/01. 10.1038/cmi.2010.67 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

