Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines
- PMID: 33259596
- PMCID: PMC7820878
- DOI: 10.1182/blood.2020008758
Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines
Abstract
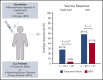
Vaccinations are effective in preventing infections; however, it is unknown if patients with chronic lymphocytic leukemia (CLL) who are treatment naïve (TN) or receiving Bruton tyrosine kinase inhibitors (BTKi's) respond to novel adjuvanted vaccines. Understanding the effect of BTKi's on humoral immunity is timely because BTKi's are widely used and vaccination against coronavirus disease 2019 is urgently needed. In 2 open-label, single-arm clinical trials, we measured the effect of BTKi's on de novo immune response against recombinant hepatitis B vaccine (HepB-CpG) and recall response against recombinant zoster vaccine (RZV) in CLL patients who were TN or on BTKi. The primary end point was serologic response to HepB-CpG (anti-hepatitis B surface antibodies ≥10 mIU/mL) and RZV (≥fourfold increase in anti-glycoprotein E). The response rate to HepB-CpG was lower in patients on BTKi (3.8%; 95% confidence interval [CI], 0.7-18.9) than patients who were TN (28.1%; 95% CI, 15.6-45.4; P = .017). In contrast, the response rate to RZV did not differ significantly between the BTKi (41.5%; 95% CI, 27.8-56.6) and TN cohorts (59.1%; 95% CI, 38.7-76.7; P = .2). BTKi's were associated with a decreased de novo immune response following HepB-CpG, whereas recall immune response following RZV was not significantly affected by BTKi therapy. These trials were registered at www.clinicaltrials.gov as #NCT03685708 (Hep-CpG) and #NCT03702231 (RZV).
Conflict of interest statement
Conflict-of-interest disclosure: A.W. received research support from Pharmacyclics LLC, an AbbVie company; Acerta Pharma, a member of the Astra-Zeneca group; Merck; Nurix; Verastem; and Genmab. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Vaccinations in CLL: implications for COVID-19.Blood. 2021 Jan 14;137(2):144-146. doi: 10.1182/blood.2020009966. Blood. 2021. PMID: 33443562 Free PMC article.
References
-
- Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: Linking mechanisms with infections. Blood Rev. 2018;32(5):387-399. - PubMed
-
- Whitaker JA, Shanafelt TD, Poland GA, Kay NE. Room for improvement: immunizations for patients with monoclonal B-cell lymphocytosis or chronic lymphocytic leukemia. Clin Adv Hematol Oncol. 2014;12(7):440-450. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical