Cellular and physiological functions of C9ORF72 and implications for ALS/FTD
- PMID: 33259633
- PMCID: PMC8842544
- DOI: 10.1111/jnc.15255
Cellular and physiological functions of C9ORF72 and implications for ALS/FTD
Abstract
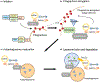
The hexanucleotide repeat expansion (HRE) in the C9ORF72 gene is the main cause of two tightly linked neurodegenerative diseases, amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). HRE leads to not only a gain of toxicity from RNA repeats and dipeptide repeats but also reduced levels of C9ORF72 protein. However, the cellular and physiological functions of C9ORF72 were unknown until recently. Through proteomic analysis, Smith-Magenis chromosome regions 8 (SMCR8) and WD repeat-containing protein (WDR41) were identified as binding partners of C9ORF72. These three proteins have been shown to form a tight complex, but the exact functions of this complex remain to be characterized. Both C9ORF72 and SMCR8 contain a DENN domain, which has been shown to regulate the activities of small GTPases. The C9ORF72 complex has been implicated in many cellular processes, including vesicle trafficking, lysosome homeostasis, mTORC1 signaling , and autophagy. C9ORF72 deficiency in mice results in exaggerated inflammatory responses and human patients with C9ORF72 mutations have neuroinflammation phenotype. Recent studies indicate that C9ORF72 regulates trafficking and lysosomal degradation of inflammatory mediators, including toll-like receptors (TLRs) and STING, to affect inflammatory outputs. Further exploration of cellular and physiological functions of C9ORF72 will help dissect the pathological mechanism of ALS/FTD caused by C9ORF72 mutations.
Keywords: ALS; C9ORF72; FTD; SMCR8; WDR41; autophagy; inflammation; lysosome; mTORC1.
© 2020 International Society for Neurochemistry.
Conflict of interest statement
CONFLIC T OF INTEREST
The authors declare that they have no conflict of interest.
Figures
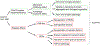


References
-
- Abo-Rady M, Kalmbach N, Pal A, Schludi C, Janosch A, Richter T, Freitag P, Bickle M, Kahlert AK, Petri S, Stefanov S, Glass H, Staege S, Just W, Bhatnagar R, Edbauer D, Hermann A, Wegner F, Sterneckert JL (2020). Knocking out C9ORF72 Exacerbates Axonal Trafficking Defects Associated with Hexanucleotide Repeat Expansion and Reduces Levels of Heat Shock Proteins. Stem Cell Reports, 14, 390–405. 10.1016/j.stemcr.2020.01.010. - DOI - PMC - PubMed
-
- Aktas O, Ullrich O, Infante-Duarte C, Nitsch R, & Zipp F (2007). Neuronal damage in brain inflammation. Archives of Neurology, 64, 185–189. - PubMed
-
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobágyi T, Shaw CE (2011). p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathologica, 122, 691–702. 10.1007/s00401-011-0911-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

