Electroreductive Olefin-Ketone Coupling
- PMID: 33259715
- PMCID: PMC8353665
- DOI: 10.1021/jacs.0c11214
Electroreductive Olefin-Ketone Coupling
Abstract
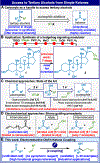
A user-friendly approach is presented to sidestep the venerable Grignard addition to unactivated ketones to access tertiary alcohols by reversing the polarity of the disconnection. In this work a ketone instead acts as a nucleophile when adding to simple unactivated olefins to accomplish the same overall transformation. The scope of this coupling is broad as enabled using an electrochemical approach, and the reaction is scalable, chemoselective, and requires no precaution to exclude air or water. Multiple applications demonstrate the simplifying nature of the reaction on multistep synthesis, and mechanistic studies point to an intuitive mechanism reminiscent of other chemical reductants such as SmI2 (which cannot accomplish the same reaction).
Figures


References
-
- For selected reviews, see: Nicolaou KC; Montagnon T. Molecules That Changed the World. Wiley-VCH; (2008);
- Arimoto H; Uemura D. In: Quaternary Stereocenters, Challenges and Solutions for Organic Synthesis; (Eds.: Christoffers J, Baro A), Wiley-VCH, Weinheim, 2005, chap.1, pp 1–24;
- de Vries JG in: Quaternary Stereocenters, Challenges and Solutions for Organic Synthesis; (Eds.: Christoffers J, Baro A), Wiley-V CH, Weinheim, 2005, chap. 2, pp 25–50.
-
- For selected reviews, see: Motwani HV; De Rosa M; Odell LR; Hallberg A; Larhed M. Aspartic protease inhibitors containing tertiary alcohol transition-state mimics. Eur. J. Med. Chem 2015, 90, 462–490; - PubMed
- Talele TT; Natural-Products-Inspired Use of the gem-Dimethyl Group in Medicinal Chemistry. J. Med. Chem 2018, 61, 2166–2210; - PubMed
- Cramer J; Sager CP; Ernst B. Hydroxyl Groups in Synthetic and Natural-Product-Derived Therapeutics: A Perspective on a Common Functional Group. J. Med. Chem 2019, 62, 8915–8930. - PubMed
-
- For selected reviews, see: Chen L; Yin X-P; Wang C-H; Zhou J. Catalytic functionalization of tertiary alcohols to fully substituted carbon centres. Org. Biomol. Chem 2014, 12, 6033–6048.; - PubMed
- Naredla RR; Klumpp DA Contemporary Carbocation Chemistry: Applications in Organic Synthesis. Chem. Rev 2013, 113, 6905–6948. - PubMed
-
- For a selected example, see: Vollhardt K; Schore N. Organic Chemistry: Structure and Function; Freeman WH: New York, 2014.
-
- For selected reviews, see: Rappoport Z, Marek I, Eds. The Chemistry of Organomagnesium Compounds; Wiley-VCH: Weinheim, Germany, 2008;
- Seyferth D. The Grignard Reagents. Organometallics 2009, 28, 1598–1605;
- Silverman GS; Rakita PE Handbook of Grignard Reagents; CRC Press: New York, 1996;
- Knochel P; Dohle W; Gommermann N; Kneisel FF; Kopp F; Korn T; Sapountzis I; Vu VA Highly Functionalized Organomagnesium Reagents Prepared through Halogen-Metal Exchange. Angew. Chem., Int. Ed 2003, 42, 4302–4320; - PubMed
- Rappoport Z; Marek I, Eds. The Chemistry of Organolithium Compounds, Wiley-VCH, 2004;
- Luisi R; Capriati V. Eds. Lithium Compounds in Organic Synthesis – From Fundamentals to Applications, Wiley-VCH, 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

