Biomimetic six-axis robots replicate human cardiac papillary muscle motion: pioneering the next generation of biomechanical heart simulator technology
- PMID: 33259750
- PMCID: PMC7811588
- DOI: 10.1098/rsif.2020.0614
Biomimetic six-axis robots replicate human cardiac papillary muscle motion: pioneering the next generation of biomechanical heart simulator technology
Abstract
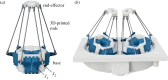
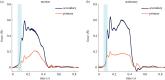
Papillary muscles serve as attachment points for chordae tendineae which anchor and position mitral valve leaflets for proper coaptation. As the ventricle contracts, the papillary muscles translate and rotate, impacting chordae and leaflet kinematics; this motion can be significantly affected in a diseased heart. In ex vivo heart simulation, an explanted valve is subjected to physiologic conditions and can be adapted to mimic a disease state, thus providing a valuable tool to quantitatively analyse biomechanics and optimize surgical valve repair. However, without the inclusion of papillary muscle motion, current simulators are limited in their ability to accurately replicate cardiac biomechanics. We developed and implemented image-guided papillary muscle (IPM) robots to mimic the precise motion of papillary muscles. The IPM robotic system was designed with six degrees of freedom to fully capture the native motion. Mathematical analysis was used to avoid singularity conditions, and a supercomputing cluster enabled the calculation of the system's reachable workspace. The IPM robots were implemented in our heart simulator with motion prescribed by high-resolution human computed tomography images, revealing that papillary muscle motion significantly impacts the chordae force profile. Our IPM robotic system represents a significant advancement for ex vivo simulation, enabling more reliable cardiac simulations and repair optimizations.
Keywords: biomechanics; cardiac imaging; ex vivo modelling; robotics.
Conflict of interest statement
We declare we have no competing interests.
Figures


References
-
- Fontaine AA, He S, Stadter R, Ellis JT, Levine RA, Yoganathan AP. 1996. In vitro assessment of prosthetic valve function in mitral valve replacement with chordal preservation techniques. J. Heart Valve Dis. 5, 186–198. - PubMed
-
- Erek E, Padala M, Pekkan K, Jimenez J, Yalçinba YK, Salihoğlu E, Sarioğlu T, Yoganathan AP. 2009. Mitral web—a new concept for mitral valve repair: improved engineering design and in-vitro studies. J. Heart Valve Dis. 18, 300–306. - PubMed
-
- Padala M, Powell SN, Croft LR, Thourani VH, Yoganathan AP, Adams DH. 2009. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J. Thorac. Cardiovasc. Surg. 138, 309–315. (10.1016/j.jtcvs.2009.01.031) - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
