COVID-19 and non-COVID ARDS patients demonstrate a distinct response to low dose steroids- A retrospective observational study
- PMID: 33260010
- PMCID: PMC7680030
- DOI: 10.1016/j.jcrc.2020.11.012
COVID-19 and non-COVID ARDS patients demonstrate a distinct response to low dose steroids- A retrospective observational study
Abstract
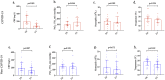
Patients with COVID-19 ARDS have distinct physiological and immunological phenotypes compared to patients with non-COVID ARDS. Patients with COVID-19 ARDS (n = 32) had a significant improvement in PaO2: FiO2 ratio (p = 0.046) following low-dose steroid treatment, unlike patients with non-COVID ARDS (n = 16) (p = 0.529). Patients with COVID-19 ARDS had a greater fall in CRP compared to patients with non-COVID ARDS, albeit not statistically significant (p = 0.07). Our novel findings highlight differences in the underlying physiological and immunological phenotypes between COVID-19 and non-COVID ARDS, with implications for future ARDS studies.
Keywords: ARDS; COVID-19; Inflammation; Steroids.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

