Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro
- PMID: 33260331
- PMCID: PMC7760070
- DOI: 10.3390/jcm9123880
Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro
Abstract
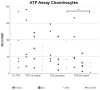
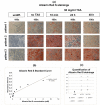
The topical application of tranexamic acid (TXA) helps to prevent post-operative blood loss in total joint replacements. Despite these findings, the effects on articular and periarticular tissues remain unclear. Therefore, this in vitro study examined the effects of varying exposure times and concentrations of TXA on proliferation rates, gene expression and differentiation capacity of chondrocytes and human mesenchymal stromal cells (hMSCs), which underwent osteogenic differentiation. Chondrocytes and hMSCs were isolated and multiplied in monolayer cell cultures. Osteogenic differentiation of hMSCs was induced for 21 days using a differentiation medium containing specific growth factors. Cell proliferation was analyzed using ATP assays. Effects of TXA on cell morphology were examined via light microscopy and histological staining, while expression levels of tissue-specific genes were measured using semiquantitative RT-PCR. After treatment with 50 mg/mL of TXA, a decrease in cell proliferation rates was observed. Furthermore, treatment with concentrations of 20 mg/mL of TXA for at least 48 h led to a visible detachment of chondrocytes. TXA treatment with 50 mg/mL for at least 24 h led to a decrease in the expression of specific marker genes in chondrocytes and osteogenically differentiated hMSCs. No significant effects were observed for concentrations beyond 20 mg/mL of TXA combined with exposure times of less than 24 h. This might therefore represent a safe limit for topical application in vivo. Further research regarding in vivo conditions and effects on hMSC functionality are necessary to fully determine the effects of TXA on articular and periarticular tissues.
Keywords: chondrocytes; differentiation capacity; hMSCs; osteoarthritis; toxicity; tranexamic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Fernandes A.C. Health at a Glance 2019. OECD Publishing; Paris, France: 2019. OECD Indicators.
LinkOut - more resources
Full Text Sources

