Role of Regular Physical Activity in Neuroprotection against Acute Ischemia
- PMID: 33260365
- PMCID: PMC7731306
- DOI: 10.3390/ijms21239086
Role of Regular Physical Activity in Neuroprotection against Acute Ischemia
Abstract
One of the major obstacles that prevents an effective therapeutic intervention against ischemic stroke is the lack of neuroprotective agents able to reduce neuronal damage; this results in frequent evolution towards a long-term disability with limited alternatives available to aid in recovery. Nevertheless, various treatment options have shown clinical efficacy. Neurotrophins such as brain-derived neurotrophic factor (BDNF), widely produced throughout the brain, but also in distant tissues such as the muscle, have demonstrated regenerative properties with the potential to restore damaged neural tissue. Neurotrophins play a significant role in both protection and recovery of function following neurological diseases such as ischemic stroke or traumatic brain injury. Unfortunately, the efficacy of exogenous administration of these neurotrophins is limited by rapid degradation with subsequent poor half-life and a lack of blood-brain-barrier permeability. Regular exercise seems to be a therapeutic approach able to induce the activation of several pathways related to the neurotrophins release. Exercise, furthermore, reduces the infarct volume in the ischemic brain and ameliorates motor function in animal models increasing astrocyte proliferation, inducing angiogenesis and reducing neuronal apoptosis and oxidative stress. One of the most critical issues is to identify the relationship between neurotrophins and myokines, newly discovered skeletal muscle-derived factors released during and after exercise able to exert several biological functions. Various myokines (e.g., Insulin-Like Growth Factor 1, Irisin) have recently shown their ability to protects against neuronal injury in cerebral ischemia models, suggesting that these substances may influence the degree of neuronal damage in part via inhibiting inflammatory signaling pathways. The aim of this narrative review is to examine the main experimental data available to date on the neuroprotective and anti-ischemic role of regular exercise, analyzing also the possible role played by neurotrophins and myokines.
Keywords: brain-derived neurotrophic factor (BDNF); inflammation; ischemic stroke; myokines; neuronal recovery; neurotrophins; physical activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


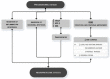
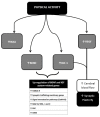
References
-
- American College of Sports Medicine . In: ACM’s Guidelines for Exercise Testing and Prescription. 10th ed. Riebe D., Ehrman J.K., Liguori G., Magal M., editors. Wolters Kluwer; Philadelphia, PA, USA: 2018.
-
- Wahid A., Manek N., Nichols M., Kelly P., Foster C., Webster P., Kaur A., Friedemann Smith C., Wilkins E., Rayner M., et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016;5:e002495. doi: 10.1161/JAHA.115.002495. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

