A New Bioassay Platform Design for the Discovery of Small Molecules with Anticancer Immunotherapeutic Activity
- PMID: 33260400
- PMCID: PMC7760914
- DOI: 10.3390/md18120604
A New Bioassay Platform Design for the Discovery of Small Molecules with Anticancer Immunotherapeutic Activity
Abstract
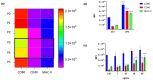
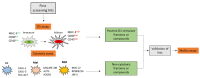
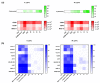
Immunotherapy takes advantage of the immune system to prevent, control, and eliminate neoplastic cells. The research in the field has already led to major breakthroughs to treat cancer. In this work, we describe a platform that integrates in vitro bioassays to test the immune response and direct antitumor effects for the preclinical discovery of anticancer candidates. The platform relies on the use of dendritic cells that are professional antigen-presenting cells (APC) able to activate T cells and trigger a primary adaptive immune response. The experimental procedure is based on two phenotypic assays for the selection of chemical leads by both a panel of nine tumor cell lines and growth factor-dependent immature mouse dendritic cells (D1). The positive hits are then validated by a secondary test on human monocyte-derived dendritic cells (MoDCs). The aim of this approach is the selection of potential immunotherapeutic small molecules from natural extracts or chemical libraries.
Keywords: anticancer; bioassay platform; chemical immunology; dendritic cell; drug discovery; high throughput; immunotherapy; screening guidelines.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Modulation of lactate-lysosome axis in dendritic cells by clotrimazole potentiates antitumor immunity.J Immunother Cancer. 2021 May;9(5):e002155. doi: 10.1136/jitc-2020-002155. J Immunother Cancer. 2021. PMID: 34016722 Free PMC article.
-
Inhibition of topoisomerase I shapes antitumor immunity through the induction of monocyte-derived dendritic cells.Cancer Lett. 2021 Nov 1;520:38-47. doi: 10.1016/j.canlet.2021.06.031. Epub 2021 Jul 3. Cancer Lett. 2021. PMID: 34224797
-
β-glucan from Aureobasidium pullulans augments the anti-tumor immune responses through activated tumor-associated dendritic cells.Int Immunopharmacol. 2021 Dec;101(Pt A):108265. doi: 10.1016/j.intimp.2021.108265. Epub 2021 Oct 29. Int Immunopharmacol. 2021. PMID: 34715491
-
Tumoricidal activity of human dendritic cells.Trends Immunol. 2014 Jan;35(1):38-46. doi: 10.1016/j.it.2013.10.007. Epub 2013 Nov 18. Trends Immunol. 2014. PMID: 24262387 Free PMC article. Review.
-
Cancer immunotherapy by dendritic cells.Immunity. 2008 Sep 19;29(3):372-83. doi: 10.1016/j.immuni.2008.08.004. Immunity. 2008. PMID: 18799145 Review.
Cited by
-
Marine natural product lepadin A as a novel inducer of immunogenic cell death via CD91-dependent pathway.Nat Prod Bioprospect. 2023 Oct 2;13(1):34. doi: 10.1007/s13659-023-00401-3. Nat Prod Bioprospect. 2023. PMID: 37779162 Free PMC article.
-
The immunoregulatory effect of the TREM2-agonist Sulfavant A in human allogeneic mixed lymphocyte reaction.Front Immunol. 2023 Feb 14;14:1050113. doi: 10.3389/fimmu.2023.1050113. eCollection 2023. Front Immunol. 2023. PMID: 36865548 Free PMC article.
-
Genome Mining as an Alternative Way for Screening the Marine Organisms for Their Potential to Produce UV-Absorbing Mycosporine-like Amino Acid.Mar Drugs. 2022 Jul 26;20(8):478. doi: 10.3390/md20080478. Mar Drugs. 2022. PMID: 35892946 Free PMC article. Review.
-
Stimulators of immunogenic cell death for cancer therapy: focusing on natural compounds.Cancer Cell Int. 2023 Sep 13;23(1):200. doi: 10.1186/s12935-023-03058-7. Cancer Cell Int. 2023. PMID: 37705051 Free PMC article. Review.
-
Bioactive Polyketides from Amphidinium spp.: An In-Depth Review of Biosynthesis, Applications, and Current Research Trends.Mar Drugs. 2025 Jun 16;23(6):255. doi: 10.3390/md23060255. Mar Drugs. 2025. PMID: 40559664 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

