Molecular Regulation of Copper Homeostasis in the Male Gonad during the Process of Spermatogenesis
- PMID: 33260507
- PMCID: PMC7730223
- DOI: 10.3390/ijms21239053
Molecular Regulation of Copper Homeostasis in the Male Gonad during the Process of Spermatogenesis
Abstract
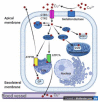
Owing to its redox properties, copper is a cofactor of enzymes that catalyze reactions in fundamental metabolic processes. However, copper-oxygen interaction, which is a source of toxic oxygen radicals generated by the Fenton reaction, makes copper a doubled-edged-sword in an oxygen environment. Among the microelements influencing male fertility, copper plays a special role because both copper deficiency and overload in the gonads worsen spermatozoa quality and disturb reproductive function in mammals. Male gametes are produced during spermatogenesis, a multi-step process that consumes large amounts of oxygen. Germ cells containing a high amount of unsaturated fatty acids in their membranes are particularly vulnerable to excess copper-mediated oxidative stress. In addition, an appropriate copper level is necessary to initiate meiosis in premeiotic germ cells. The balance between essential and toxic copper concentrations in germ cells at different stages of spermatogenesis and in Sertoli cells that support their development is handled by a network of copper importers, chaperones, recipient proteins, and exporters. Here, we describe coordinated regulation/functioning of copper-binding proteins expressed in germ and Sertoli cells with special emphasis on copper transporters, copper transporting ATPases, and SOD1, a copper-dependent antioxidant enzyme. These and other proteins assure copper bioavailability in germ cells and protection against copper toxicity.
Keywords: ATP7A; ATP7B; CTR1; copper; gametes production; spermatogenesis; testis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Atp7a and Atp7b regulate copper homeostasis in developing male germ cells in mice.Metallomics. 2017 Sep 20;9(9):1288-1303. doi: 10.1039/c7mt00134g. Metallomics. 2017. PMID: 28820536
-
Iron and copper in male reproduction: a double-edged sword.J Assist Reprod Genet. 2015 Jan;32(1):3-16. doi: 10.1007/s10815-014-0344-7. Epub 2014 Sep 23. J Assist Reprod Genet. 2015. PMID: 25245929 Free PMC article. Review.
-
Molecular machinery providing copper bioavailability for spermatozoa along the epididymial tubule in mouse.Biol Reprod. 2019 Jun 1;100(6):1505-1520. doi: 10.1093/biolre/ioz028. Biol Reprod. 2019. PMID: 30997485
-
Role of copper in the process of spermatogenesis.Postepy Hig Med Dosw (Online). 2017 Aug 9;71(0):663-683. doi: 10.5604/01.3001.0010.3846. Postepy Hig Med Dosw (Online). 2017. PMID: 28791960 Review.
-
Testicular toxicity and sperm quality following copper exposure in Wistar albino rats: ameliorative potentials of L-carnitine.Environ Sci Pollut Res Int. 2018 Jan;25(2):1837-1862. doi: 10.1007/s11356-017-0624-8. Epub 2017 Nov 4. Environ Sci Pollut Res Int. 2018. PMID: 29103113
Cited by
-
Whole Exome Sequencing and In Silico Analysis of Human Sertoli in Patients with Non-Obstructive Azoospermia.Int J Mol Sci. 2022 Oct 20;23(20):12570. doi: 10.3390/ijms232012570. Int J Mol Sci. 2022. PMID: 36293429 Free PMC article.
-
[Gandou Bushen Decoction improves spermatogenesis and promotes spermatogenic cell proliferation in Wilson disease TX mice by activating testicular ERK signaling pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Nov 20;44(11):2063-2073. doi: 10.12122/j.issn.1673-4254.2024.11.02. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39623261 Free PMC article. Chinese.
-
Relative Abundance of Spermadhesin-1 in the Seminal Plasma of Young Nellore Bulls Is in Agreement with Reproductive Parameters.Vet Sci. 2023 Oct 7;10(10):610. doi: 10.3390/vetsci10100610. Vet Sci. 2023. PMID: 37888562 Free PMC article.
-
Antioxidant Intervention against Male Infertility: Time to Design Novel Strategies.Biomedicines. 2022 Nov 28;10(12):3058. doi: 10.3390/biomedicines10123058. Biomedicines. 2022. PMID: 36551814 Free PMC article. Review.
-
Identification and immuno-infiltration analysis of cuproptosis regulators in human spermatogenic dysfunction.Front Genet. 2023 Mar 29;14:1115669. doi: 10.3389/fgene.2023.1115669. eCollection 2023. Front Genet. 2023. PMID: 37065492 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

