Effects of Biological Therapies on Molecular Features of Rheumatoid Arthritis
- PMID: 33260629
- PMCID: PMC7731249
- DOI: 10.3390/ijms21239067
Effects of Biological Therapies on Molecular Features of Rheumatoid Arthritis
Abstract
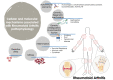
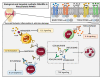
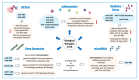
Rheumatoid arthritis (RA) is an autoimmune and chronic inflammatory disease primarily affecting the joints, and closely related to specific autoantibodies that mostly target modified self-epitopes. Relevant findings in the field of RA pathogenesis have been described. In particular, new insights come from studies on synovial fibroblasts and cells belonging to the innate and adaptive immune system, which documented the aberrant production of inflammatory mediators, oxidative stress and NETosis, along with relevant alterations of the genome and on the regulatory epigenetic mechanisms. In recent years, the advances in the understanding of RA pathogenesis by identifying key cells and cytokines allowed the development of new targeted disease-modifying antirheumatic drugs (DMARDs). These drugs considerably improved treatment outcomes for the majority of patients. Moreover, numerous studies demonstrated that the pharmacological therapy with biologic DMARDs (bDMARDs) promotes, in parallel to their clinical efficacy, significant improvement in all these altered molecular mechanisms. Thus, continuous updating of the knowledge of molecular processes associated with the pathogenesis of RA, and on the specific effects of bDMARDs in the correction of their dysregulation, are essential in the early and correct approach to the treatment of this complex autoimmune disorder. The present review details basic mechanisms related to the physiopathology of RA, along with the core mechanisms of response to bDMARDs.
Keywords: NETosis; autoimmunity; bDMARDs; epigenetic and pos-transcriptional mechanisms; genome; inflammation; oxidative stress; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

