Function and Regulation of the Pyruvate Transporter CstA in Escherichia coli
- PMID: 33260635
- PMCID: PMC7730263
- DOI: 10.3390/ijms21239068
Function and Regulation of the Pyruvate Transporter CstA in Escherichia coli
Abstract
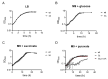
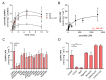
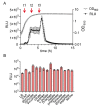
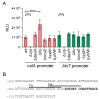
Pyruvate is a central metabolite that connects many metabolic pathways in living organisms. To meet the cellular pyruvate requirements, the enterobacterium Escherichia coli has at least three pyruvate uptake systems-the H+/pyruvate symporter BtsT, and two thus far less well-characterized transporters, YhjX and CstA. BtsT and CstA belong to the putative carbon starvation (CstA) family (transporter classification TC# 2.A.114). We have created an E. coli mutant that cannot grow on pyruvate as the sole carbon source and used it to characterize CstA as a pyruvate transporter. Transport studies in intact cells confirmed that CstA is a highly specific pyruvate transporter with moderate affinity and is energized by a proton gradient. When cells of a reporter strain were cultured in complex medium, cstA expression was maximal only in stationary phase. A DNA affinity-capture assay combined with mass spectrometry and an in-vivo reporter assay identified Fis as a repressor of cstA expression, in addition to the known activator cAMP-CRP. The functional characterization and regulation of this second pyruvate uptake system provides valuable information for understanding the complexity of pyruvate sensing and uptake in E. coli.
Keywords: catabolite repression; global regulator Fis; pyruvate uptake; secondary transporter; stationary phase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Peptide Transporter CstA Imports Pyruvate in Escherichia coli K-12.J Bacteriol. 2018 Mar 12;200(7):e00771-17. doi: 10.1128/JB.00771-17. Print 2018 Apr 1. J Bacteriol. 2018. PMID: 29358499 Free PMC article.
-
BtsT, a Novel and Specific Pyruvate/H+ Symporter in Escherichia coli.J Bacteriol. 2017 Dec 20;200(2):e00599-17. doi: 10.1128/JB.00599-17. Print 2018 Jan 15. J Bacteriol. 2017. PMID: 29061664 Free PMC article.
-
CsrA regulates translation of the Escherichia coli carbon starvation gene, cstA, by blocking ribosome access to the cstA transcript.J Bacteriol. 2003 Aug;185(15):4450-60. doi: 10.1128/JB.185.15.4450-4460.2003. J Bacteriol. 2003. PMID: 12867454 Free PMC article.
-
Characterization of pyruvate uptake in Escherichia coli K-12.PLoS One. 2013 Jun 20;8(6):e67125. doi: 10.1371/journal.pone.0067125. Print 2013. PLoS One. 2013. PMID: 23818977 Free PMC article.
-
Pyruvate transport systems in organelles: future directions in C4 biology research.Curr Opin Plant Biol. 2016 Jun;31:143-8. doi: 10.1016/j.pbi.2016.04.007. Epub 2016 May 3. Curr Opin Plant Biol. 2016. PMID: 27153467 Review.
Cited by
-
Insights into a Pyruvate Sensing and Uptake System in Vibrio campbellii and Its Importance for Virulence.J Bacteriol. 2021 Sep 23;203(20):e0029621. doi: 10.1128/JB.00296-21. Epub 2021 Aug 2. J Bacteriol. 2021. PMID: 34339295 Free PMC article.
-
The EcoCyc Database in 2021.Front Microbiol. 2021 Jul 28;12:711077. doi: 10.3389/fmicb.2021.711077. eCollection 2021. Front Microbiol. 2021. PMID: 34394059 Free PMC article.
-
Serine Deamination Is a New Acid Tolerance Mechanism Observed in Uropathogenic Escherichia coli.mBio. 2022 Dec 20;13(6):e0296322. doi: 10.1128/mbio.02963-22. Epub 2022 Dec 5. mBio. 2022. PMID: 36468870 Free PMC article.
-
Genetic dissection of the degradation pathways for the mycotoxin fusaric acid in Burkholderia ambifaria T16.Appl Environ Microbiol. 2023 Dec 21;89(12):e0063023. doi: 10.1128/aem.00630-23. Epub 2023 Dec 6. Appl Environ Microbiol. 2023. PMID: 38054732 Free PMC article.
-
The Biological Significance of Pyruvate Sensing and Uptake in Salmonella enterica Serovar Typhimurium.Microorganisms. 2022 Aug 30;10(9):1751. doi: 10.3390/microorganisms10091751. Microorganisms. 2022. PMID: 36144354 Free PMC article.
References
-
- Schär J., Stoll R., Schauer K., Loeffler D.I.M., Eylert E., Joseph B., Eisenreich W., Fuchs T.M., Goebel W. Pyruvate carboxylase plays a crucial role in carbon metabolism of extra and intracellularly replicating Listeria monocytogenes. J. Bacteriol. 2010;192:1774–1784. doi: 10.1128/JB.01132-09. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

