High-Density Lipoprotein Modifications: A Pathological Consequence or Cause of Disease Progression?
- PMID: 33260660
- PMCID: PMC7759904
- DOI: 10.3390/biomedicines8120549
High-Density Lipoprotein Modifications: A Pathological Consequence or Cause of Disease Progression?
Abstract
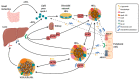
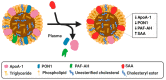
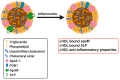
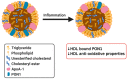
High-density lipoprotein (HDL) is well-known for its cardioprotective effects, as it possesses anti-inflammatory, anti-oxidative, anti-thrombotic, and cytoprotective properties. Traditionally, studies and therapeutic approaches have focused on raising HDL cholesterol levels. Recently, it became evident that, not HDL cholesterol, but HDL composition and functionality, is probably a more fruitful target. In disorders, such as chronic kidney disease or cardiovascular diseases, it has been observed that HDL is modified and becomes dysfunctional. There are different modification that can occur, such as serum amyloid, an enrichment and oxidation, carbamylation, and glycation of key proteins. Additionally, the composition of HDL can be affected by changes to enzymes such as cholesterol ester transfer protein (CETP), lecithin-cholesterol acyltransferase (LCAT), and phospholipid transfer protein (PLTP) or by modification to other important components. This review will highlight some main modifications to HDL and discuss whether these modifications are purely a consequential result of pathology or are actually involved in the pathology itself and have a causal role. Therefore, HDL composition may present a molecular target for the amelioration of certain diseases, but more information is needed to determine to what extent HDL modifications play a causal role in disease development.
Keywords: HDL modifications; dysfunctional HDL; high-density lipoproteins; inflammation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Mahley R.W., Innerarity T.L., Rall S.C., Jr., Weisgraber K.H. Plasma lipoproteins: Apolipoprotein structure and function. J. Lipid Res. 1984;25:1277–1294. - PubMed
-
- Gordon D.J., Probstfield J.L., Garrison R.J., Neaton J.D., Castelli W.P., Knoke J.D., Jacobs D.R., Jr., Bangdiwala S., Tyroler H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.CIR.79.1.8. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

