Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats
- PMID: 33260714
- PMCID: PMC7760003
- DOI: 10.3390/nu12123661
Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats
Abstract
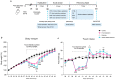
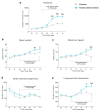
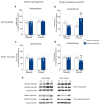
Intense physical activity and dieting are core symptoms of anorexia nervosa (AN). Their combination evolves into compulsivity, leading the patient into an out-of-control spiral. AN patients exhibit an altered activation of nucleus accumbens (NAc), revealing a dysfunctional mesocorticolimbic reward circuitry in AN. Since evidence exists that a dysregulation of the glutamate system in the NAc influences reward and taking advantage of the activity-based anorexia (ABA) rat model, which closely mimics the hallmarks of AN, we investigated the involvement of the glutamatergic signaling in the NAc in this experimental model. We here demonstrate that food restriction causes hyperactive and compulsive behavior in rodents, inducing an escalation of physical activity, which results in dramatic weight loss. Analysis of the glutamate system revealed that, in the acute phase of the pathology, ABA rats increased the membrane expression of GluA1 AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor subunits together with its scaffolding protein SAP97. Recovery of body weight reduced GluN2A/2B balance together with the expression of their specific scaffolding proteins, thus suggesting persistent maladaptive neurotransmission. Taken together, AMPA and NMDA (N-methyl-D-aspartate) receptor subunit reorganization may play a role in the motivational mechanisms underlying AN.
Keywords: activity-based anorexia; adolescence; female rats; glutamatergic receptors; nucleus accumbens; reward circuit.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Cortical reorganization of the glutamate synapse in the activity-based anorexia rat model: Impact on cognition.J Neurochem. 2022 May;161(4):350-365. doi: 10.1111/jnc.15605. Epub 2022 Mar 25. J Neurochem. 2022. PMID: 35257377 Free PMC article.
-
Brief exposure to enriched environment rapidly shapes the glutamate synapses in the rat brain: A metaplastic fingerprint.Eur J Neurosci. 2024 Mar;59(5):982-995. doi: 10.1111/ejn.16279. Epub 2024 Feb 20. Eur J Neurosci. 2024. PMID: 38378276
-
Ethanol Experience Enhances Glutamatergic Ventral Hippocampal Inputs to D1 Receptor-Expressing Medium Spiny Neurons in the Nucleus Accumbens Shell.J Neurosci. 2019 Mar 27;39(13):2459-2469. doi: 10.1523/JNEUROSCI.3051-18.2019. Epub 2019 Jan 28. J Neurosci. 2019. PMID: 30692226 Free PMC article.
-
Stimulation of N-methyl-D-aspartate receptors, AMPA receptors or metabotropic glutamate receptors leads to rapid internalization of AMPA receptors in cultured nucleus accumbens neurons.Eur J Neurosci. 2004 Aug;20(3):649-57. doi: 10.1111/j.1460-9568.2004.03511.x. Eur J Neurosci. 2004. PMID: 15255976
-
Molecular Plasticity of the Nucleus Accumbens Revisited-Astrocytic Waves Shall Rise.Mol Neurobiol. 2019 Dec;56(12):7950-7965. doi: 10.1007/s12035-019-1641-z. Epub 2019 May 27. Mol Neurobiol. 2019. PMID: 31134458 Free PMC article. Review.
Cited by
-
Long-lasting BDNF signaling alterations in the amygdala of adolescent female rats exposed to the activity-based anorexia model.Front Behav Neurosci. 2022 Dec 8;16:1087075. doi: 10.3389/fnbeh.2022.1087075. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36570702 Free PMC article.
-
From Desire to Dread-A Neurocircuitry Based Model for Food Avoidance in Anorexia Nervosa.J Clin Med. 2021 May 21;10(11):2228. doi: 10.3390/jcm10112228. J Clin Med. 2021. PMID: 34063884 Free PMC article. Review.
-
Activity-based anorexia animal model: a review of the main neurobiological findings.J Eat Disord. 2021 Oct 2;9(1):123. doi: 10.1186/s40337-021-00481-x. J Eat Disord. 2021. PMID: 34600568 Free PMC article. Review.
-
Cortical reorganization of the glutamate synapse in the activity-based anorexia rat model: Impact on cognition.J Neurochem. 2022 May;161(4):350-365. doi: 10.1111/jnc.15605. Epub 2022 Mar 25. J Neurochem. 2022. PMID: 35257377 Free PMC article.
-
The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa.J Clin Med. 2021 Dec 30;11(1):186. doi: 10.3390/jcm11010186. J Clin Med. 2021. PMID: 35011927 Free PMC article. Review.
References
-
- Sullivan P.F. Mortality in anorexia nervosa. Am. J. Psychiatry. 1995;152:1073–1074. - PubMed

