Synthesis of Nanoscale Liposomes via Low-Cost Microfluidic Systems
- PMID: 33260732
- PMCID: PMC7760644
- DOI: 10.3390/mi11121050
Synthesis of Nanoscale Liposomes via Low-Cost Microfluidic Systems
Abstract
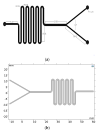
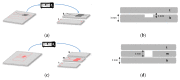
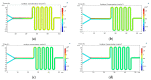
We describe the manufacture of low-cost microfluidic systems to produce nanoscale liposomes with highly uniform size distributions (i.e., low polydispersity indexes (PDI)) and acceptable colloidal stability. This was achieved by exploiting a Y-junction device followed by a serpentine micromixer geometry to facilitate the diffusion between the mixing phases (i.e., continuous and dispersed) via advective processes. Two different geometries were studied. In the first one, the microchannels were engraved with a laser cutting machine on a polymethyl methacrylate (PMMA) sheet and covered with another PMMA sheet to form a two-layer device. In the second one, microchannels were not engraved but through-hole cut on a PMMA sheet and encased by a top and a bottom PMMA sheet to form a three-layer device. The devices were tested out by putting in contact lipids dissolved in alcohol as the dispersed phase and water as the continuous phase to self-assemble the liposomes. By fixing the total flow rate (TFR) and varying the flow rate ratio (FRR), we obtained most liposomes with average hydrodynamic diameters ranging from 188 ± 61 to 1312 ± 373 nm and 0.30 ± 0.09 PDI values. Such liposomes were obtained by changing the FRR from 5:1 to 2:1. Our results approached those obtained by conventional bulk synthesis methods such as a thin hydration bilayer and freeze-thaw, which produced liposomes with diameters ranging from 200 ± 38 to 250 ± 38 nm and 0.30 ± 0.05 PDI values. The produced liposomes might find several potential applications in the biomedical field, particularly in encapsulation and drug delivery.
Keywords: liposomes; low-cost; microfluidic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
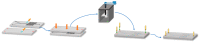


References
Grants and funding
LinkOut - more resources
Full Text Sources

