Optimization of Enzyme Essays to Enhance Reliability of Activity Measurements in Leukocyte Lysates for the Diagnosis of Metachromatic Leukodystrophy and Gangliosidoses
- PMID: 33260765
- PMCID: PMC7761145
- DOI: 10.3390/cells9122553
Optimization of Enzyme Essays to Enhance Reliability of Activity Measurements in Leukocyte Lysates for the Diagnosis of Metachromatic Leukodystrophy and Gangliosidoses
Abstract
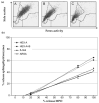
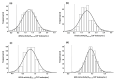
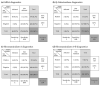
(1) Lysosomal storage diseases are rare inherited disorders with no standardized or commercially available tests for biochemical diagnosis. We present factors influencing the quality of enzyme assays for metachromatic leukodystrophy (MLD) and gangliosidoses (GM1; GM2 variants B and 0) and validate the reliability and stability of testing in a retrospective analysis of 725 samples. (2) Patient leukocytes were isolated from ethylene-diamine-tetra-acetic acid (EDTA) blood and separated for subpopulation experiments using density gradient centrifugation or magnetic cell separation. Enzyme activities in whole leukocyte lysate and leukocyte subpopulations were determined. (3) The enzyme activities in leukocyte subpopulations differed significantly. Compared to lymphocytes, the respective enzyme activities were 2.31-4.57-fold higher in monocytes and 1.64-2.81-fold higher in granulocytes. During sample preparation, a considerable amount of the lysosomal enzymes was released from granulocytes. Nevertheless, with the sample preparation method used here, total leukocyte count proved to be more accurate than total protein amount as a reference unit for enzyme activities. Subsequent analysis of 725 individuals showed clear discrimination of enzyme activities in patient samples (48 MLD; 21 gangliosidoses), with a sensitivity of 100% and specificity of 98-99%.
Keywords: gangliosidoses; lysosomal storage disease; metachromatic leukodystrophy; sphingolipidoses.
Conflict of interest statement
S.G. received institutional research support from Shire/Takeda, outside of the submitted work. He is an advisor and co-investigator for trials in Metachromatic Leukodystrophy (Shire/Takeda, Orchard, Bioclinica, Homology Medicine), but receives no personal payment related to this role. I.K.-M. received travel funds from shire and acts as advisor for Orchard. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Mehta A.B., Winchester B. Lysosomal Storage Disorders: A Practical Guide. 1st ed. John Wiley & Sons; Hoboken, NJ, USA: 2012.
-
- Bouwman M.G., Teunissen Q.G.A., Wijburg F.A., Linthorst G.E. ‘Doctor Google’ ending the diagnostic odyssey in lysosomal storage disorders: Parents using internet search engines as an efficient diagnostic strategy in rare diseases. Arch. Dis. Child. 2010;95:642–644. doi: 10.1136/adc.2009.171827. - DOI - PubMed
-
- Groeschel S., Kühl J.-S., Bley A.E., Kehrer C., Weschke B., Döring M., Böhringer J., Schrum J., Santer R., Kohlschütter A., et al. Long-term Outcome of Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Juvenile Metachromatic Leukodystrophy Compared With Nontransplanted Control Patients. JAMA Neurol. 2016;73:1133–1140. doi: 10.1001/jamaneurol.2016.2067. - DOI - PubMed
-
- Dali C.Í., Sevin C., Krägeloh-Mann I., Giugliani R., Sakai N., Wu J., Wasilewski M. Safety of intrathecal delivery of recombinant human arylsulfatase A in children with metachromatic leukodystrophy: Results from a phase 1/2 clinical trial. Mol. Genet. Metab. 2020 doi: 10.1016/j.ymgme.2020.07.002. - DOI - PubMed
-
- Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A., et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: An ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

