Resolved Influenza A Virus Infection Has Extended Effects on Lung Homeostasis and Attenuates Allergic Airway Inflammation in a Mouse Model
- PMID: 33260910
- PMCID: PMC7761027
- DOI: 10.3390/microorganisms8121878
Resolved Influenza A Virus Infection Has Extended Effects on Lung Homeostasis and Attenuates Allergic Airway Inflammation in a Mouse Model
Abstract
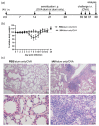
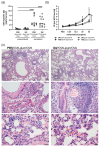
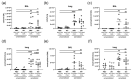
Allergic airway inflammation (AAI) involves T helper cell type 2 (Th2) and pro-inflammatory responses to aeroallergens and many predisposing factors remain elusive. Influenza A virus (IAV) is a major human pathogen that causes acute respiratory infections and induces specific immune responses essential for viral clearance and resolution of the infection. Beyond acute infection, IAV has been shown to persistently affect lung homeostasis and respiratory immunity. Here we asked how resolved IAV infection affects subsequently induced AAI. Mice infected with a sublethal dose of IAV were sensitized and challenged in an ovalbumin mediated mouse model for AAI after resolution of the acute viral infection. Histological changes, respiratory leukocytes, cytokines and airway hyperreactivity were analyzed in resolved IAV infection alone and in AAI with and without previous IAV infection. More than five weeks after infection, we detected persistent pneumonia with increased activated CD4+ and CD8+ lymphocytes as well as dendritic cells and MHCII expressing macrophages in the lung. Resolved IAV infection significantly affected subsequently induced AAI on different levels including morphological changes, respiratory leukocytes and lymphocytes as well as the pro-inflammatory cytokine responses, which was clearly diminished. We conclude that IAV has exceptional persisting effects on respiratory immunity with substantial consequences for subsequently induced AAI.
Keywords: allergic airway inflammation; allergic asthma; influenza A virus; macrophages; pro-inflammatory cytokines; respiratory immune regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

