1-(3- Tert-Butylphenyl)-2,2,2-Trifluoroethanone as a Potent Transition-State Analogue Slow-Binding Inhibitor of Human Acetylcholinesterase: Kinetic, MD and QM/MM Studies
- PMID: 33260981
- PMCID: PMC7760592
- DOI: 10.3390/biom10121608
1-(3- Tert-Butylphenyl)-2,2,2-Trifluoroethanone as a Potent Transition-State Analogue Slow-Binding Inhibitor of Human Acetylcholinesterase: Kinetic, MD and QM/MM Studies
Abstract
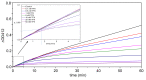
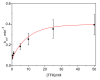
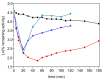
Kinetic studies and molecular modeling of human acetylcholinesterase (AChE) inhibition by a fluorinated acetophenone derivative, 1-(3-tert-butylphenyl)-2,2,2-trifluoroethanone (TFK), were performed. Fast reversible inhibition of AChE by TFK is of competitive type with Ki = 5.15 nM. However, steady state of inhibition is reached slowly. Kinetic analysis showed that TFK is a slow-binding inhibitor (SBI) of type B with Ki* = 0.53 nM. Reversible binding of TFK provides a long residence time, τ = 20 min, on AChE. After binding, TFK acylates the active serine, forming an hemiketal. Then, disruption of hemiketal (deacylation) is slow. AChE recovers full activity in approximately 40 min. Molecular docking and MD simulations depicted the different steps. It was shown that TFK binds first to the peripheral anionic site. Then, subsequent slow induced-fit step enlarged the gorge, allowing tight adjustment into the catalytic active site. Modeling of interactions between TFK and AChE active site by QM/MM showed that the "isomerization" step of enzyme-inhibitor complex leads to a complex similar to substrate tetrahedral intermediate, a so-called "transition state analog", followed by a labile covalent intermediate. SBIs of AChE show prolonged pharmacological efficacy. Thus, this fluoroalkylketone intended for neuroimaging, could be of interest in palliative therapy of Alzheimer's disease and protection of central AChE against organophosphorus compounds.
Keywords: acetylcholinesterase; organophosphorus; slow-binding inhibition; transition state analog.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Slow-binding inhibition of acetylcholinesterase by an alkylammonium derivative of 6-methyluracil: mechanism and possible advantages for myasthenia gravis treatment.Biochem J. 2016 May 1;473(9):1225-36. doi: 10.1042/BCJ20160084. Epub 2016 Feb 29. Biochem J. 2016. PMID: 26929400
-
Protective effects of m-(tert-butyl) trifluoroacetophenone, a transition state analogue of acetylcholine, against paraoxon toxicity and memory impairments.Chem Biol Interact. 2021 Aug 25;345:109558. doi: 10.1016/j.cbi.2021.109558. Epub 2021 Jun 17. Chem Biol Interact. 2021. PMID: 34147486
-
α-tocopherol, a slow-binding inhibitor of acetylcholinesterase.Chem Biol Interact. 2021 Oct 1;348:109646. doi: 10.1016/j.cbi.2021.109646. Epub 2021 Sep 8. Chem Biol Interact. 2021. PMID: 34506764
-
Molecular interaction of human brain acetylcholinesterase with a natural inhibitor huperzine-B: an enzoinformatics approach.CNS Neurol Disord Drug Targets. 2014 Apr;13(3):487-90. doi: 10.2174/18715273113126660163. CNS Neurol Disord Drug Targets. 2014. PMID: 24059299 Review.
-
Slow-binding inhibitors of acetylcholinesterase of medical interest.Neuropharmacology. 2020 Oct 15;177:108236. doi: 10.1016/j.neuropharm.2020.108236. Epub 2020 Jul 23. Neuropharmacology. 2020. PMID: 32712274 Review.
Cited by
-
Cholinesterase Research.Biomolecules. 2021 Jul 30;11(8):1121. doi: 10.3390/biom11081121. Biomolecules. 2021. PMID: 34439787 Free PMC article.
-
Drug Design in the Exascale Era: A Perspective from Massively Parallel QM/MM Simulations.J Chem Inf Model. 2023 Jun 26;63(12):3647-3658. doi: 10.1021/acs.jcim.3c00557. Epub 2023 Jun 15. J Chem Inf Model. 2023. PMID: 37319347 Free PMC article. Review.
-
New Multifunctional Agents for Potential Alzheimer's Disease Treatment Based on Tacrine Conjugates with 2-Arylhydrazinylidene-1,3-Diketones.Biomolecules. 2022 Oct 24;12(11):1551. doi: 10.3390/biom12111551. Biomolecules. 2022. PMID: 36358901 Free PMC article.
References
-
- Nair H.K., Lee K., Quinn D.M. m-(N,N,N-Trimethylammonio)trifluoroacetophenone: A femtomolar inhibitor of acetylcholinesterase. J. Am. Chem. Soc. 1993;115:9939–9941. doi: 10.1021/ja00075a009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

