Implications of ABCC4-Mediated cAMP Eflux for CRC Migration
- PMID: 33261018
- PMCID: PMC7760996
- DOI: 10.3390/cancers12123547
Implications of ABCC4-Mediated cAMP Eflux for CRC Migration
Abstract
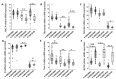
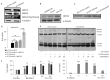
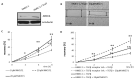
Colorectal cancer (CRC) presents significant molecular heterogeneity. The cellular plasticity of epithelial to mesenchymal transition (EMT) is one of the key factors responsible for the heterogeneous nature of metastatic CRC. EMT is an important regulator of ATP binding cassette (ABC) protein expression; these proteins are the active transporters of a broad range of endogenous compounds and anticancer drugs. In our previous studies, we performed a transcriptomic and functional analysis of CRC in the early stages of metastasis induced by the overexpression of Snail, the transcription factor involved in EMT initiation. Interestingly, we found a correlation between the Snail expression and ABCC4 (MRP4) protein upregulation. The relationship between epithelial transition and ABCC4 expression and function in CRC has not been previously defined. In the current study, we propose that the ABCC4 expression changes during EMT and may be differentially regulated in various subpopulations of CRC. We confirmed that ABCC4 upregulation is correlated with the phenotype conversion process in CRC. The analysis of Gene Expression Omnibus (GEO) sets showed that the ABCC4 expression was elevated in CRC patients. The results of a functional study demonstrated that, in CRC, ABCC4 can regulate cell migration in a cyclic nucleotide-dependent manner.
Keywords: ABC transporters; ABCC4 protein; colon cancer; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The xenobiotic transporter ABCC4/MRP4 promotes epithelial mesenchymal transition in pancreatic cancer.Front Pharmacol. 2024 Jul 24;15:1432851. doi: 10.3389/fphar.2024.1432851. eCollection 2024. Front Pharmacol. 2024. PMID: 39114357 Free PMC article.
-
Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression.BMC Cancer. 2015 Mar 5;15:97. doi: 10.1186/s12885-015-1119-y. BMC Cancer. 2015. PMID: 25884904 Free PMC article.
-
Characteristics of ABCC4 and ABCG2 High Expression Subpopulations in CRC-A New Opportunity to Predict Therapy Response.Cancers (Basel). 2023 Nov 28;15(23):5623. doi: 10.3390/cancers15235623. Cancers (Basel). 2023. PMID: 38067326 Free PMC article.
-
Snail/FOXK1/Cyr61 Signaling Axis Regulates the Epithelial-Mesenchymal Transition and Metastasis in Colorectal Cancer.Cell Physiol Biochem. 2018;47(2):590-603. doi: 10.1159/000490015. Epub 2018 May 22. Cell Physiol Biochem. 2018. PMID: 29794466
-
CAPS1 promotes colorectal cancer metastasis via Snail mediated epithelial mesenchymal transformation.Oncogene. 2019 Jun;38(23):4574-4589. doi: 10.1038/s41388-019-0740-7. Epub 2019 Feb 11. Oncogene. 2019. PMID: 30742066
Cited by
-
The xenobiotic transporter ABCC4/MRP4 promotes epithelial mesenchymal transition in pancreatic cancer.Front Pharmacol. 2024 Jul 24;15:1432851. doi: 10.3389/fphar.2024.1432851. eCollection 2024. Front Pharmacol. 2024. PMID: 39114357 Free PMC article.
-
Isothiocyanates (ITCs) 1-(Isothiocyanatomethyl)-4-phenylbenzene and 1-Isothiocyanato-3,5-bis(trifluoromethyl)benzene-Aldehyde Dehydrogenase (ALDH) Inhibitors, Decreases Cisplatin Tolerance and Migratory Ability of NSCLC.Int J Mol Sci. 2022 Aug 3;23(15):8644. doi: 10.3390/ijms23158644. Int J Mol Sci. 2022. PMID: 35955773 Free PMC article.
-
Extracellular Vesicles and Cancer Multidrug Resistance: Undesirable Intercellular Messengers?Life (Basel). 2023 Jul 27;13(8):1633. doi: 10.3390/life13081633. Life (Basel). 2023. PMID: 37629489 Free PMC article. Review.
-
An Approach to Cell Motility as a Key Mechanism in Oncology.Cancers (Basel). 2021 Jul 16;13(14):3576. doi: 10.3390/cancers13143576. Cancers (Basel). 2021. PMID: 34298789 Free PMC article.
-
Integrated Bioinformatics Analysis of the Hub Genes Involved in Irinotecan Resistance in Colorectal Cancer.Biomedicines. 2022 Jul 16;10(7):1720. doi: 10.3390/biomedicines10071720. Biomedicines. 2022. PMID: 35885025 Free PMC article.
References
-
- Souza e Silva V., Chinen L.T.D., Abdallah E.A., Damascena A., Paludo J., Chojniak R., Dettino A.L.A., de Mello C.A.L., Alves V.S., Fanelli M.F. Early detection of poor outcome in patients with metastatic colorectal cancer: Tumor kinetics evaluated by circulating tumor cells. Onco Targets Ther. 2016;9:7503–7513. doi: 10.2147/OTT.S115268. - DOI - PMC - PubMed
-
- Jolly M.K., Somarelli J.A., Sheth M., Biddle A., Tripathi S.C., Armstrong A.J., Hanash S.M., Bapat S.A., Rangarajan A., Levine H. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. Ther. 2019;194:161–184. doi: 10.1016/j.pharmthera.2018.09.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

