Cyclodextrin-Based Contrast Agents for Medical Imaging
- PMID: 33261035
- PMCID: PMC7730728
- DOI: 10.3390/molecules25235576
Cyclodextrin-Based Contrast Agents for Medical Imaging
Abstract
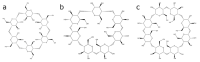
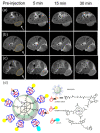
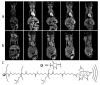
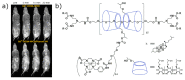
Cyclodextrins (CDs) are naturally occurring cyclic oligosaccharides consisting of multiple glucose subunits. CDs are widely used in host-guest chemistry and biochemistry due to their structural advantages, biocompatibility, and ability to form inclusion complexes. Recently, CDs have become of high interest in the field of medical imaging as a potential scaffold for the development of a large variety of the contrast agents suitable for magnetic resonance imaging, ultrasound imaging, photoacoustic imaging, positron emission tomography, single photon emission computed tomography, and computed tomography. The aim of this review is to summarize and highlight the achievements in the field of cyclodextrin-based contrast agents for medical imaging.
Keywords: CT; MRI; PAI; PET; SPECT; contrast agents; medical imaging; α-cyclodextrin; β-cyclodextrin; γ-cyclodextrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Del Valle E.M.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004;39:1033–1046. doi: 10.1016/S0032-9592(03)00258-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

