Molecular Dissection of Cucumis metuliferus Resistance against Papaya Ringspot Virus by Grafting
- PMID: 33261122
- PMCID: PMC7759848
- DOI: 10.3390/plants9121666
Molecular Dissection of Cucumis metuliferus Resistance against Papaya Ringspot Virus by Grafting
Abstract
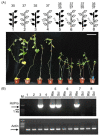
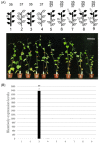
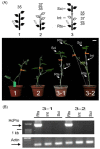
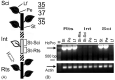
Vegetable crops of the genus Cucumis are very popular worldwide and have great market value. However, their fruit quality and yield are hindered by viral diseases. C. metuliferus is considered a wild species with resistance to viral diseases that is lacking in cultivated crops of the Cucumis genus, such as melon. The C. metuliferus line L37 shows extreme resistance against Papaya ringspot virus (PRSV-HA), whereas line L35 is a susceptible line. In this study, reciprocal grafting experiments between L35 and L37 were performed, and the PRSV-HA strain was pre-inoculated in the rootstock leaves. The results revealed that the resistance signal in the L37 rootstock could transmit and provide resistance to the L35 scion. Subsequently, double sandwich grafting was performed using the pre-inoculated L35 as the rootstock, which was then grafted onto the L37 intermediate and the L35 scion. The results showed that PRSV-HA RNA accumulated in the L35 rootstock leaf, petiole, and stem tissues, whereas PRSV-HA RNA accumulated in some intermediate and scion petiole and stem tissues. No HCPro RNA was detected in the L35 scion leaves. The results showed that the suppression of the virus occurred in the leaves, and the resistance effect spread from the rootstock in the scion direction. Hence, this study has demonstrated that RNA silencing of systemic signals is responsible for L37 resistance against PRSV. C. metuliferus L37 could provide a valuable resistance source for crops of the Cucumis species against viral diseases through grafting.
Keywords: Cucumis metuliferus; RNA silencing; grafting; immune; virus resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lim T.K. Cucumis metuliferus. In: Lim T.K., editor. Edible Medicinal and Non-Medicinal Plants. Volume 2. Springer; Dordrecht, The Netherlands: 2012. pp. 235–238.
-
- Nisini P.T., Buzi A., Granati E., Chilosi G., Crinò P., Magro P. Screening for resistance to Didymella bryoniae in rootstocks of melon. EPPO Bull. 2000;30:231–234. doi: 10.1111/j.1365-2338.2000.tb00885.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

