Diagnosis, Classification and Management of Mast Cell Activation Syndromes (MCAS) in the Era of Personalized Medicine
- PMID: 33261124
- PMCID: PMC7731385
- DOI: 10.3390/ijms21239030
Diagnosis, Classification and Management of Mast Cell Activation Syndromes (MCAS) in the Era of Personalized Medicine
Abstract
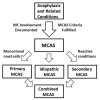
Mast cell activation (MCA) is seen in a variety of clinical contexts and pathologies, including IgE-dependent allergic inflammation, other immunologic and inflammatory reactions, primary mast cell (MC) disorders, and hereditary alpha tryptasemia (HAT). MCA-related symptoms range from mild to severe to life-threatening. The severity of MCA-related symptoms depends on a number of factors, including genetic predisposition, the number and releasability of MCs, organs affected, and the type and consequences of comorbid conditions. In severe systemic reactions, MCA is demonstrable by a substantial increase of basal serum tryptase levels above the individual's baseline. When, in addition, the symptoms are recurrent, involve more than one organ system, and are responsive to therapy with MC-stabilizing or mediator-targeting drugs, the consensus criteria for the diagnosis of MCA syndrome (MCAS) are met. Based on the etiology of MCA, patients can further be classified as having i) primary MCAS where KIT-mutated, clonal, MCs are detected; ii) secondary MCAS where an underlying IgE-dependent allergy or other reactive MCA-triggering pathology is found; or iii) idiopathic MCAS, where neither a triggering reactive state nor KIT-mutated MCs are identified. Most severe MCA events occur in combined forms of MCAS, where KIT-mutated MCs, IgE-dependent allergies and sometimes HAT are detected. These patients may suffer from life-threatening anaphylaxis and are candidates for combined treatment with various types of drugs, including IgE-blocking antibodies, anti-mediator-type drugs and MC-targeting therapy. In conclusion, detailed knowledge about the etiology, underlying pathologies and co-morbidities is important to establish the diagnosis and develop an optimal management plan for MCAS, following the principles of personalized medicine.
Keywords: Hereditary alpha tryptasemia; IgE; Mast cell activation syndrome; Mastocytosis.
Conflict of interest statement
The authors declare that they have no conflict of interest in this study. LBS: VCU receives royalties from Thermo Fisher for the tryptase assay that are shared with its inventor, LBS.
Figures
References
-
- Schwartz L.B. Mast cells and basophils. Clin. Allergy Immunol. 2002;16:3–42. - PubMed
-
- Valent P., Akin C., Hartmann K., Nilsson G., Reiter A., Hermine O., Sotlar K., Sperr W.R., Escribano L., George T.I., et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics. 2020;10:10743–10768. doi: 10.7150/thno.46719. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous