Hodgkin Reed-Sternberg-Like Cells in Non-Hodgkin Lymphoma
- PMID: 33261174
- PMCID: PMC7760963
- DOI: 10.3390/diagnostics10121019
Hodgkin Reed-Sternberg-Like Cells in Non-Hodgkin Lymphoma
Abstract
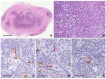
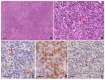
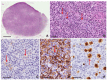
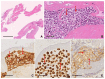
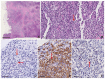
Reed-Sternberg cells (RSCs) are hallmarks of classic Hodgkin lymphoma (cHL). However, cells with a similar morphology and immunophenotype, so-called Reed-Sternberg-like cells (RSLCs), are occasionally seen in both B cell and T cell non-Hodgkin Lymphomas (NHLs). In NHLs, RSLCs are usually present as scattered elements or in small clusters, and the typical background microenviroment of cHL is usually absent. Nevertheless, in NHLs, the phenotype of RSLCs is very similar to typical RSCs, staining positive for CD30 and EBV, and often for B cell lineage markers, and negative for CD45/LCA. Due to different therapeutic approaches and prognostication, it is mandatory to distinguish between cHL and NHLs. Herein, NHL types in which RSLCs can be detected along with clinicopathological correlation are described. Moreover, the main helpful clues in the differential diagnosis with cHL are summarized.
Keywords: B cell lymphoma; CD30; Reed-Sternberg cell; Reed-Sternberg-like cell; T cell lymphoma; classic Hodgkin Lymphoma; lymphoma diagnosis; lymphoma diagnostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

