Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles
- PMID: 33261219
- PMCID: PMC7759840
- DOI: 10.3390/pharmaceutics12121156
Recent Advances and Challenges in Controlling the Spatiotemporal Release of Combinatorial Anticancer Drugs from Nanoparticles
Abstract
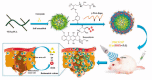
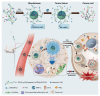
To overcome cancer, various chemotherapeutic studies are in progress; among these, studies on nano-formulated combinatorial drugs (NFCDs) are being actively pursued. NFCDs function via a fusion technology that includes a drug delivery system using nanoparticles as a carrier and a combinatorial drug therapy using two or more drugs. It not only includes the advantages of these two technologies, such as ensuring stability of drugs, selectively transporting drugs to cancer cells, and synergistic effects of two or more drugs, but also has the additional benefit of enabling the spatiotemporal and controlled release of drugs. This spatial and temporal drug release from NFCDs depends on the application of nanotechnology and the composition of the combination drug. In this review, recent advances and challenges in the control of spatiotemporal drug release from NFCDs are provided. To this end, the types of combinatorial drug release for various NFCDs are classified in terms of time and space, and the detailed programming techniques used for this are described. In addition, the advantages of the time and space differences in drug release in terms of anticancer efficacy are introduced in depth.
Keywords: controlled release; nano-formulated combinatorial drug; ratiometric; sequential; spatiotemporal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

