Serological evidence of human infections with highly pathogenic avian influenza A(H5N1) virus: a systematic review and meta-analysis
- PMID: 33261599
- PMCID: PMC7709391
- DOI: 10.1186/s12916-020-01836-y
Serological evidence of human infections with highly pathogenic avian influenza A(H5N1) virus: a systematic review and meta-analysis
Abstract
Background: Highly pathogenic avian influenza A(H5N1) virus poses a global public health threat given severe and fatal zoonotic infections since 1997 and ongoing A(H5N1) virus circulation among poultry in several countries. A comprehensive assessment of the seroprevalence of A(H5N1) virus antibodies remains a gap and limits understanding of the true risk of A(H5N1) virus infection.
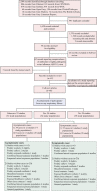
Methods: We conducted a systematic review and meta-analysis of published serosurveys to assess the risk of subclinical and clinically mild A(H5N1) virus infections. We assessed A(H5N1) virus antibody titers and changes in titers among populations with variable exposures to different A(H5N1) viruses.
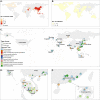
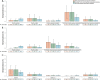
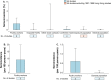
Results: Across studies using the World Health Organization-recommended seropositive definition, the point estimates of the seroprevalence of A(H5N1) virus-specific antibodies were higher in poultry-exposed populations (range 0-0.6%) and persons exposed to both human A(H5N1) cases and infected birds (range 0.4-1.8%) than in close contacts of A(H5N1) cases or the general population (none to very low frequencies). Seroprevalence was higher in persons exposed to A(H5N1) clade 0 virus (1.9%, range 0.7-3.2%) than in participants exposed to other clades of A(H5N1) virus (range 0-0.5%) (p < 0.05). Seroprevalence was higher in poultry-exposed populations (range 0-1.9%) if such studies utilized antigenically similar A(H5N1) virus antigens in assays to A(H5N1) viruses circulating among poultry.
Conclusions: These low seroprevalences suggest that subclinical and clinically mild human A(H5N1) virus infections are uncommon. Standardized serological survey and laboratory methods are needed to fully understand the extent and risk of human A(H5N1) virus infections.
Keywords: Influenza A(H5N1); Influenza in humans; Serological evidence.
Conflict of interest statement
H.Y. has received research funding from Sanofi Pasteur, and Shanghai Roche Pharmaceutical Company; none of this research funding is related to avian influenza viruses. BJC has received honoraria from Roche and Sanofi Pasteur. X.C., W.W., Y.W., S.L., J.Y., B.J.C., P.W.H., and T.M.U. declare no competing interests.
Figures

References
-
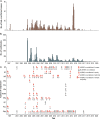
- Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2019 [https://www.who.int/influenza/human_animal_interface/2019_09_27_tableH5N...].
-
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza AV, Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N et al. Update on avian influenza A (H5N1) virus infection in humans. New England J Med 2008, 358(3):261–273. - PubMed
Publication types
MeSH terms
Grants and funding
- 82073613/National Natural Science Foundation of China/International
- 2018ZX10201001-007/National Science and Technology Major project of China/International
- 2018ZX10201001-010/National Science and Technology Major project of China/International
- 18XD1400300/Program of Shanghai Academic/Technology Research Leader/International
- 81525023/National Science Fund for Distinguished Young Scholars/International
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

