Altered striatal dopamine levels in Parkinson's disease VPS35 D620N mutant transgenic aged mice
- PMID: 33261640
- PMCID: PMC7706192
- DOI: 10.1186/s13041-020-00704-3
Altered striatal dopamine levels in Parkinson's disease VPS35 D620N mutant transgenic aged mice
Abstract
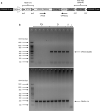
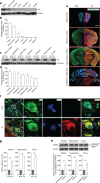
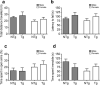
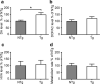
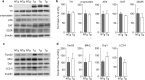
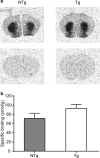
Vacuolar protein sorting 35 (VPS35) is a major component of the retromer complex that mediates the retrograde transport of cargo proteins from endosomes to the trans-Golgi network. Mutations such as D620N in the VPS35 gene have been identified in patients with autosomal dominant Parkinson's disease (PD). However, it remains poorly understood whether and how VPS35 deficiency or mutation contributes to PD pathogenesis; specifically, the studies that have examined VPS35 thus far have differed in results and methodologies. We generated a VPS35 D620N mouse model using a Rosa26-based transgene expression platform to allow expression in a spatial manner, so as to better address these discrepancies. Here, aged (20-months-old) mice were first subjected to behavioral tests. Subsequently, DAB staining analysis of substantia nigra (SN) dopaminergic neurons with the marker for tyrosine hydroxylase (TH) was performed. Next, HPLC was used to determine dopamine levels, along with levels of its two metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), in the striatum. Western blotting was also performed to study the levels of key proteins associated with PD. Lastly, autoradiography (ARG) evaluation of [3H]FE-PE2I binding to the striatal dopamine transporter DAT was carried out. We found that VPS35 D620N Tg mice displayed a significantly higher dopamine level than NTg counterparts. All results were then compared with that of current VPS35 studies to shed light on the disease pathogenesis. Our model allows future studies to explicitly control spatial expression of the transgene which would generate a more reliable PD phenotype.
Keywords: Behavioral assay; Parkinson’s disease; Striatal dopamine; Transgenic mice; VPS35 D620N.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

