Limited Specificity of Serologic Tests for SARS-CoV-2 Antibody Detection, Benin
- PMID: 33261717
- PMCID: PMC7774555
- DOI: 10.3201/eid2701.203281
Limited Specificity of Serologic Tests for SARS-CoV-2 Antibody Detection, Benin
Abstract
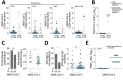
We used commercially available ELISAs to test 68 samples from coronavirus disease cases and prepandemic controls from Benin. We noted <25% false-positive results among controls, likely due to unspecific immune responses elicited by acute malaria. Serologic tests must be carefully evaluated to assess coronavirus disease spread and immunity in tropical regions.
Keywords: Benin; COVID-19; SARS; SARS-CoV-2; West Africa; coronavirus; coronavirus disease; malaria; parasites; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

