Drug repurposing for the treatment of COVID-19: Pharmacological aspects and synthetic approaches
- PMID: 33261844
- PMCID: PMC7676325
- DOI: 10.1016/j.bioorg.2020.104488
Drug repurposing for the treatment of COVID-19: Pharmacological aspects and synthetic approaches
Abstract
In December 2019, a new variant of SARS-CoV emerged, the so-called acute severe respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus causes the new coronavirus disease (COVID-19) and has been plaguing the world owing to its unprecedented spread efficiency, which has resulted in a huge death toll. In this sense, the repositioning of approved drugs is the fastest way to an effective response to a pandemic outbreak of this scale. Considering these facts, in this review we provide a comprehensive and critical discussion on the chemical aspects surrounding the drugs currently being studied as candidates for COVID-19 therapy. We intend to provide the general chemical community with an overview on the synthetic/biosynthetic pathways related to such molecules, as well as their mechanisms of action against the evaluated viruses and some insights on the pharmacological interactions involved in each case. Overall, the review aims to present the chemical aspects of the main bioactive molecules being considered to be repositioned for effective treatment of COVID-19 in all phases, from the mildest to the most severe.
Keywords: Coronavirus; Organic synthesis; SARS-CoV-2; Small molecules; Therapy.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




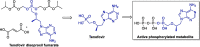


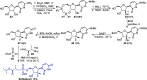
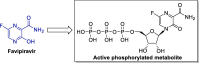






















References
-
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J.Y. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Eng. J. Med. 2003;348:1986–1994. - PubMed
-
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Eng. J. Med. 2012;367:1814–1820. - PubMed
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. - PMC - PubMed
-
- COVID-19 Coronavirus pandemic. Available at https://www.worldometers.info/coronavirus/ (accessed in June 27th, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

