The immunology of SARS-CoV-2 infections and vaccines
- PMID: 33262067
- PMCID: PMC7670910
- DOI: 10.1016/j.smim.2020.101422
The immunology of SARS-CoV-2 infections and vaccines
Abstract
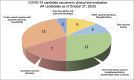
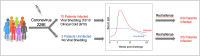
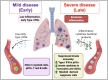
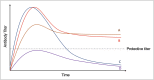
SARS-CoV-2, the virus that causes COVID-19, emerged in late 2019, and was declared a global pandemic on March 11th 2020. With over 50 million cases and 1.2 million deaths around the world, to date, this pandemic represents the gravest global health crisis of our times. Thus, the race to develop a COVID-19 vaccine is an urgent global imperative. At the time of writing, there are over 165 vaccine candidates being developed, with 33 in various stages of clinical testing. In this review, we discuss emerging insights about the human immune response to SARS-CoV-2, and their implications for vaccine design. We then review emerging knowledge of the immunogenicity of the numerous vaccine candidates that are currently being tested in the clinic and discuss the range of immune defense mechanisms that can be harnessed to develop novel vaccines that confer durable protection against SARS-CoV-2. Finally, we conclude with a discussion of the potential role of a systems vaccinology approach in accelerating the clinical testing of vaccines, to meet the urgent needs posed by the pandemic.
Keywords: COVID-19; Systems vaccinology; Vaccines.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- WHO . 2020. Draft Landscape of COVID-19 Candidate Vaccines.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... (Accessed 09/21/2020 2020)
-
- Mulligan M.J. Phase 1/2 study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

