Deep Neural Network for Reducing the Screening Workload in Systematic Reviews for Clinical Guidelines: Algorithm Validation Study
- PMID: 33262102
- PMCID: PMC7806440
- DOI: 10.2196/22422
Deep Neural Network for Reducing the Screening Workload in Systematic Reviews for Clinical Guidelines: Algorithm Validation Study
Abstract
Background: Performing systematic reviews is a time-consuming and resource-intensive process.
Objective: We investigated whether a machine learning system could perform systematic reviews more efficiently.
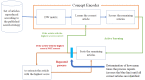
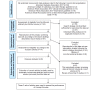
Methods: All systematic reviews and meta-analyses of interventional randomized controlled trials cited in recent clinical guidelines from the American Diabetes Association, American College of Cardiology, American Heart Association (2 guidelines), and American Stroke Association were assessed. After reproducing the primary screening data set according to the published search strategy of each, we extracted correct articles (those actually reviewed) and incorrect articles (those not reviewed) from the data set. These 2 sets of articles were used to train a neural network-based artificial intelligence engine (Concept Encoder, Fronteo Inc). The primary endpoint was work saved over sampling at 95% recall (WSS@95%).
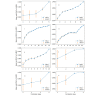
Results: Among 145 candidate reviews of randomized controlled trials, 8 reviews fulfilled the inclusion criteria. For these 8 reviews, the machine learning system significantly reduced the literature screening workload by at least 6-fold versus that of manual screening based on WSS@95%. When machine learning was initiated using 2 correct articles that were randomly selected by a researcher, a 10-fold reduction in workload was achieved versus that of manual screening based on the WSS@95% value, with high sensitivity for eligible studies. The area under the receiver operating characteristic curve increased dramatically every time the algorithm learned a correct article.
Conclusions: Concept Encoder achieved a 10-fold reduction of the screening workload for systematic review after learning from 2 randomly selected studies on the target topic. However, few meta-analyses of randomized controlled trials were included. Concept Encoder could facilitate the acquisition of evidence for clinical guidelines.
Keywords: clinical guideline; deep learning; evidence-based medicine; machine learning; meta-analysis; neural network; systematic review.
©Tomohide Yamada, Daisuke Yoneoka, Yuta Hiraike, Kimihiro Hino, Hiroyoshi Toyoshiba, Akira Shishido, Hisashi Noma, Nobuhiro Shojima, Toshimasa Yamauchi. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 30.12.2020.
Conflict of interest statement
Conflicts of Interest: HT, AS, and KH are employees of Fronteo Inc. The other authors declare no competing interests.
Figures

References
-
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996 Jan 13;312(7023):71–2. doi: 10.1136/bmj.312.7023.71. http://europepmc.org/abstract/MED/8555924 - DOI - PMC - PubMed
-
- Mulrow CD. Rationale for systematic reviews. BMJ. 1994 Sep 03;309(6954):597–9. doi: 10.1136/bmj.309.6954.597. http://europepmc.org/abstract/MED/8086953 - DOI - PMC - PubMed
-
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Training. [2018-09-01]. http://handbook.cochrane.org/
-
- Jaidee W, Moher D, Laopaiboon M. Time to update and quantitative changes in the results of Cochrane pregnancy and childbirth reviews. PLoS One. 2010 Jul 13;5(7):e11553. doi: 10.1371/journal.pone.0011553. http://dx.plos.org/10.1371/journal.pone.0011553 - DOI - PMC - PubMed
-
- Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010 Sep 21;7(9):e1000326. doi: 10.1371/journal.pmed.1000326. http://dx.plos.org/10.1371/journal.pmed.1000326 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

