PerSort Facilitates Characterization and Elimination of Persister Subpopulation in Mycobacteria
- PMID: 33262242
- PMCID: PMC7716392
- DOI: 10.1128/mSystems.01127-20
PerSort Facilitates Characterization and Elimination of Persister Subpopulation in Mycobacteria
Abstract
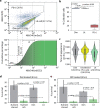
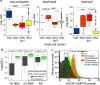
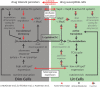
Mycobacterium tuberculosis (MTB) generates phenotypic diversity to persist and survive the harsh conditions encountered during infection. MTB avoids immune effectors and antibacterial killing by entering into distinct physiological states. The surviving cells, persisters, are a major barrier to the timely and relapse-free treatment of tuberculosis (TB). We present for the first time, PerSort, a method to isolate and characterize persisters in the absence of antibiotic or other pressure. We demonstrate the value of PerSort to isolate translationally dormant cells that preexisted in small numbers within Mycobacterium species cultures growing under optimal conditions but that dramatically increased in proportion under stress conditions. The translationally dormant subpopulation exhibited multidrug tolerance and regrowth properties consistent with those of persister cells. Furthermore, PerSort enabled single-cell transcriptional profiling that provided evidence that the translationally dormant persisters were generated through a variety of mechanisms, including vapC30, mazF, and relA/spoT overexpression. Finally, we demonstrate that notwithstanding the varied mechanisms by which the persister cells were generated, they converge on a similar low-oxygen metabolic state that was reversed through activation of respiration to rapidly eliminate persisters fostered under host-relevant stress conditions. We conclude that PerSort provides a new tool to study MTB persisters, enabling targeted strategies to improve and shorten the treatment of TB.IMPORTANCE Mycobacterium tuberculosis (MTB) persists and survives antibiotic treatments by generating phenotypically heterogeneous drug-tolerant subpopulations. The surviving cells, persisters, are a major barrier to the relapse-free treatment of tuberculosis (TB), which is already killing >1.8 million people every year and becoming deadlier with the emergence of multidrug-resistant strains. This study describes PerSort, a cell sorting method to isolate and characterize, without antibiotic treatment, translationally dormant persisters that preexist in small numbers within Mycobacterium cultures. Characterization of this subpopulation has discovered multiple mechanisms by which mycobacterial persisters emerge and unveiled the physiological basis for their dormant and multidrug-tolerant physiological state. This analysis has discovered that activating oxygen respiratory physiology using l-cysteine eliminates preexisting persister subpopulations, potentiating rapid antibiotic killing of mycobacteria under host-relevant stress. PerSort serves as a new tool to study MTB persisters for enabling targeted strategies to improve and shorten the treatment of TB.
Keywords: Mycobacterium; antibiotic tolerance; nutrient starvation; persisters; phenotypic heterogeneity.
Copyright © 2020 Srinivas et al.
Figures



References
-
- Tuberculosis Coalition for Technical Assistance. 2006. International Standards for Tuberculosis Care (ISTC). Tuberculosis Coalition for Technical Assistance, The Hague, Netherlands.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

