Sec71 separates Golgi stacks in Drosophila S2 cells
- PMID: 33262309
- PMCID: PMC10668125
- DOI: 10.1242/jcs.245571
Sec71 separates Golgi stacks in Drosophila S2 cells
Abstract
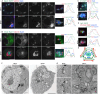
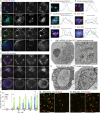
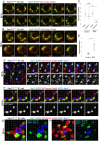
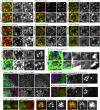
Golgi stacks are the basic structural units of the Golgi. Golgi stacks are separated from each other and scattered in the cytoplasm of Drosophila cells. Here, we report that the ARF-GEF inhibitor Brefeldin A (BFA) induces the formation of BFA bodies, which are aggregates of Golgi stacks, trans-Golgi networks and recycling endosomes. Recycling endosomes are located in the centers of BFA bodies, while Golgi stacks surround them on their trans sides. Live imaging of S2 cells revealed that Golgi stacks repeatedly merged and separated on their trans sides, and BFA caused successive merger by inhibiting separation, forming BFA bodies. S2 cells carrying genome-edited BFA-resistant mutant Sec71M717L did not form BFA bodies at high concentrations of BFA; S2 cells carrying genome-edited BFA-hypersensitive mutant Sec71F713Y produced BFA bodies at low concentrations of BFA. These results indicate that Sec71 is the sole BFA target for BFA body formation and controls Golgi stack separation. Finally, we showed that impairment of Sec71 in fly photoreceptors induces BFA body formation, with accumulation of both apical and basolateral cargoes, resulting in inhibition of polarized transport.
Keywords: Brefeldin A; Drosophila; Golgi ribbon; Recycling endosome; Sec71; Trans-Golgi network.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




Similar articles
-
Golgi clustering by the deficiency of COPI-SNARE in Drosophila photoreceptors.Front Cell Dev Biol. 2024 Sep 4;12:1442198. doi: 10.3389/fcell.2024.1442198. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39296936 Free PMC article.
-
BEN3/BIG2 ARF GEF is Involved in Brefeldin A-Sensitive Trafficking at the trans-Golgi Network/Early Endosome in Arabidopsis thaliana.Plant Cell Physiol. 2017 Oct 1;58(10):1801-1811. doi: 10.1093/pcp/pcx118. Plant Cell Physiol. 2017. PMID: 29016942
-
BFA-induced compartments from the Golgi apparatus and trans-Golgi network/early endosome are distinct in plant cells.Plant J. 2009 Dec;60(5):865-81. doi: 10.1111/j.1365-313X.2009.04007.x. Epub 2009 Aug 26. Plant J. 2009. PMID: 19709389
-
The trans-Golgi Network and the Golgi Stacks Behave Independently During Regeneration After Brefeldin A Treatment in Tobacco BY-2 Cells.Plant Cell Physiol. 2017 Apr 1;58(4):811-821. doi: 10.1093/pcp/pcx028. Plant Cell Physiol. 2017. PMID: 28339924
-
Effect of brefeldin A on the structure of the Golgi apparatus and on the synthesis and secretion of proteins and polysaccharides in sycamore maple (Acer pseudoplatanus) suspension-cultured cells.Plant Physiol. 1993 Apr;101(4):1363-73. doi: 10.1104/pp.101.4.1363. Plant Physiol. 1993. PMID: 8310065 Free PMC article.
Cited by
-
Golgi clustering by the deficiency of COPI-SNARE in Drosophila photoreceptors.Front Cell Dev Biol. 2024 Sep 4;12:1442198. doi: 10.3389/fcell.2024.1442198. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39296936 Free PMC article.
-
The yeast endocytic early/sorting compartment exists as an independent sub-compartment within the trans-Golgi network.Elife. 2023 Jul 21;12:e84850. doi: 10.7554/eLife.84850. Elife. 2023. PMID: 37477116 Free PMC article.
-
Intracellular traffic and polarity in brain development.Front Neurosci. 2023 Oct 4;17:1172016. doi: 10.3389/fnins.2023.1172016. eCollection 2023. Front Neurosci. 2023. PMID: 37859764 Free PMC article. Review.
-
HEATR5B associates with dynein-dynactin and promotes motility of AP1-bound endosomal membranes.EMBO J. 2023 Dec 1;42(23):e114473. doi: 10.15252/embj.2023114473. Epub 2023 Oct 24. EMBO J. 2023. PMID: 37872872 Free PMC article.
-
RudLOV is an optically synchronized cargo transport method revealing unexpected effects of dynasore.EMBO Rep. 2025 Feb;26(3):613-634. doi: 10.1038/s44319-024-00342-z. Epub 2024 Dec 10. EMBO Rep. 2025. PMID: 39658747 Free PMC article.
References
-
- Baluška, F., Hlavacka, A., Šamaj, J., Palme, K., Robinson, D. G., Matoh, T., McCurdy, D. W., Menzel, D. and Volkmann, D. (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130, 422-431. 10.1104/pp.007526 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

