Plasmodium falciparum sexual parasites regulate infected erythrocyte permeability
- PMID: 33262483
- PMCID: PMC7708629
- DOI: 10.1038/s42003-020-01454-7
Plasmodium falciparum sexual parasites regulate infected erythrocyte permeability
Abstract
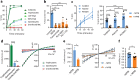
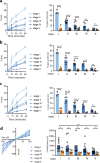
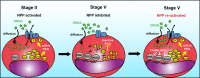
To ensure the transport of nutrients necessary for their survival, Plasmodium falciparum parasites increase erythrocyte permeability to diverse solutes. These new permeation pathways (NPPs) have been extensively characterized in the pathogenic asexual parasite stages, however the existence of NPPs has never been investigated in gametocytes, the sexual stages responsible for transmission to mosquitoes. Here, we show that NPPs are still active in erythrocytes infected with immature gametocytes and that this activity declines along gametocyte maturation. Our results indicate that NPPs are regulated by cyclic AMP (cAMP) signaling cascade, and that the decrease in cAMP levels in mature stages results in a slowdown of NPP activity. We also show that NPPs facilitate the uptake of artemisinin derivatives and that phosphodiesterase (PDE) inhibitors can reactivate NPPs and increase drug uptake in mature gametocytes. These processes are predicted to play a key role in P. falciparum gametocyte biology and susceptibility to antimalarials.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

