Selective Modulation of α5 GABAA Receptors Exacerbates Aberrant Inhibition at Key Hippocampal Neuronal Circuits in APP Mouse Model of Alzheimer's Disease
- PMID: 33262690
- PMCID: PMC7686552
- DOI: 10.3389/fncel.2020.568194
Selective Modulation of α5 GABAA Receptors Exacerbates Aberrant Inhibition at Key Hippocampal Neuronal Circuits in APP Mouse Model of Alzheimer's Disease
Abstract
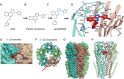
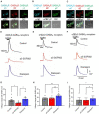
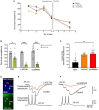
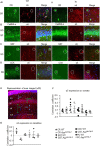
Selective negative allosteric modulators (NAMs), targeting α5 subunit-containing GABAA receptors (GABAARs) as potential therapeutic targets for disorders associated with cognitive deficits, including Alzheimer's disease (AD), continually fail clinical trials. We investigated whether this was due to the change in the expression of α5 GABAARs, consequently altering synaptic function during AD pathogenesis. Using medicinal chemistry and computational modeling, we developed aqueous soluble hybrids of 6,6-dimethyl-3-(2-hydroxyethyl) thio-1-(thiazol-2-yl)-6,7-dihydro-2-benzothiophene-4(5H)-one, that demonstrated selective binding and high negative allosteric modulation, specifically for the α5 GABAAR subtypes in constructed HEK293 stable cell-lines. Using a knock-in mouse model of AD (APP NL-F/NL-F), which expresses a mutant form of human amyloid-β (Aβ), we performed immunofluorescence studies combined with electrophysiological whole-cell recordings to investigate the effects of our key molecule, α5-SOP002 in the hippocampal CA1 region. In aged APP NL-F/NL-F mice, selective preservation of α5 GABAARs was observed in, calretinin- (CR), cholecystokinin- (CCK), somatostatin- (SST) expressing interneurons, and pyramidal cells. Previously, we reported that CR dis-inhibitory interneurons, specialized in regulating other interneurons displayed abnormally high levels of synaptic inhibition in the APP NL-F/NL-F mouse model, here we show that this excessive inhibition was "normalized" to control values with bath-applied α5-SOP002 (1 μM). However, α5-SOP002, further impaired inhibition onto CCK and pyramidal cells that were already largely compromised by exhibiting a deficit of inhibition in the AD model. In summary, using a multi-disciplinary approach, we show that exposure to α5 GABAAR NAMs may further compromise aberrant synapses in AD. We, therefore, suggest that the α5 GABAAR is not a suitable therapeutic target for the treatment of AD or other cognitive deficits due to the widespread neuronal-networks that use α5 GABAARs.
Keywords: Alzheimer’s disease; GABAA receptors; hippocampus; interneurons; synaptic.
Copyright © 2020 Petrache, Khan, Nicholson, Monaco, Kuta-Siejkowska, Haider, Hilton, Jovanovic and Ali.
Figures


References
-
- Araujo F., Ruano D., Vitorica J. (1999). Native γ-aminobutyric acid type A receptors from rat hippocampus, containing both α1 and α5 subunits, exhibit a single benzodiazepine binding site with α5 pharmacological properties. J. Pharmacol. Exp. Ther. 290, 989–997. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

