Characterization of the Blood Brain Barrier Disruption in the Photothrombotic Stroke Model
- PMID: 33262704
- PMCID: PMC7688466
- DOI: 10.3389/fphys.2020.586226
Characterization of the Blood Brain Barrier Disruption in the Photothrombotic Stroke Model
Abstract
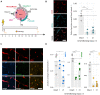
Blood brain barrier (BBB) damage is an important pathophysiological feature of ischemic stroke which significantly contributes to development of severe brain injury and therefore is an interesting target for therapeutic intervention. A popular permanent occlusion model to study long term recovery following stroke is the photothrombotic model, which so far has not been anatomically characterized for BBB leakage beyond the acute phase. Here, we observed enhanced BBB permeability over a time course of 3 weeks in peri-infarct and core regions of the ischemic cortex. Slight increases in BBB permeability could also be seen in the contralesional cortex, hippocampus and the cerebellum at different time points, regions where lesion-induced degeneration of pathways is prominent. Severe damage of tight and adherens junctions and loss of pericytes was observed within the peri-infarct region. Overall, the photothrombotic stroke model reproduces a variety of features observed in human stroke and thus, represents a suitable model to study BBB damage and therapeutic approaches interfering with this process.
Keywords: BBB; edema; ischemia; leakage; pericytes; photothrombotic stroke; tight junctions.
Copyright © 2020 Weber, Grönnert, Mulders, Maurer, Tackenberg, Schwab and Rust.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

