Simple Questionnaires to Improve Pooling Strategies for SARS-CoV-2 Laboratory Testing
- PMID: 33262937
- PMCID: PMC7678556
- DOI: 10.5334/aogh.3126
Simple Questionnaires to Improve Pooling Strategies for SARS-CoV-2 Laboratory Testing
Abstract
Background: Liberal PCR testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is key to contain the coronavirus disease 2019 (COVID-19) pandemic. Combined multi-sample testing in pools instead of single tests might enhance laboratory capacity and reduce costs, especially in low- and middle-income countries.
Objective: The purpose of our study was to assess the value of a simple questionnaire to guide and further improve pooling strategies for SARS-CoV-2 laboratory testing.
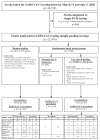
Methods: Pharyngeal swabs for SARS-CoV-2 testing were obtained from healthcare and police staff, hospital inpatients, and nursing home residents in the southwestern part of Germany. We designed a simple questionnaire, which included questions pertaining to a suggestive clinical symptomatology, recent travel history, and contact with confirmed cases to stratify an individual's pre-test probability of having contracted COVID-19. The questionnaire was adapted repeatedly in face of the unfolding pandemic in response to the evolving epidemiology and observed clinical symptomatology. Based on the response patterns, samples were either tested individually or in multi-sample pools. We compared the pool positivity rate and the number of total PCR tests required to obtain individual results between this questionnaire-based pooling strategy and randomly assembled pools.
Findings: Between March 11 and July 5, 2020, we processed 25,978 samples using random pooling (n = 6,012; 23.1%) or questionnaire-based pooling (n = 19,966; 76.9%). The overall prevalence of SARS-CoV-2 was 0.9% (n = 238). Pool positivity (14.6% vs. 1.2%) and individual SARS-CoV-2 prevalence (3.4% vs. 0.1%) were higher in the random pooling group than in the questionnaire group. The average number of PCR tests needed to obtain the individual result for one participant was 0.27 tests in the random pooling group, as compared to 0.09 in the questionnaire-based pooling group, leading to a laboratory capacity increase of 73% and 91%, respectively, as compared to single PCR testing.
Conclusions: Strategies that combine pool testing with a questionnaire-based risk stratification can increase laboratory testing capacities for COVID-19 and might be important tools, particularly in resource-constrained settings.
Copyright: © 2020 The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures
References
-
- Johns Hopkins University Coronavirus Resource Center. Coronavirus Covid-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University 2020. https://coronavirus.jhu.edu/map.html. Accessed October 20 November 16, 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous