Multicentre feasibility of multiple-breath washout in preschool children with cystic fibrosis and other lung diseases
- PMID: 33263048
- PMCID: PMC7682699
- DOI: 10.1183/23120541.00408-2020
Multicentre feasibility of multiple-breath washout in preschool children with cystic fibrosis and other lung diseases
Abstract
Background: Multiple-breath washout (MBW)-derived lung clearance index (LCI) detects early cystic fibrosis (CF) lung disease. LCI was used as an end-point in single- and multicentre settings at highly experienced MBW centres in preschool children. However, multicentre feasibility of MBW in children aged 2-6 years, including centres naïve to this technique, has not been determined systematically.
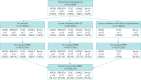
Methods: Following central training, 91 standardised nitrogen MBW investigations were performed in 74 awake preschool children (15 controls, 46 with CF, and 13 with other lung diseases), mean age 4.6±0.9 years at investigation, using a commercially available device across five centres in Germany (three experienced, two naïve to the performance in awake preschool children) with central data analysis. Each MBW investigation consisted of several measurements.
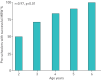
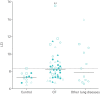
Results: Overall success rate of MBW investigations was 82.4% ranging from 70.6% to 94.1% across study sites. The number of measurements per investigation was significantly different between sites ranging from 3.7 to 6.2 (p<0.01), while the mean number of successful measurements per investigation was comparable with 2.1 (range, 1.9 to 2.5; p=0.46). In children with CF, the LCI was increased (median 8.2, range, 6.7-15.5) compared to controls (median 7.3, range 6.5-8.3; p<0.01), and comparable to children with other lung diseases (median 7.9, range, 6.6-13.9; p=0.95).
Conclusion: This study demonstrates that multicentre MBW in awake preschool children is feasible, even in centres previously naïve, with central coordination to assure standardised training, quality control and supervision. Our results support the use of LCI as multicentre end-point in clinical trials in awake preschoolers with CF.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: M. Stahl reports grants from Mukoviszidose eV, personal fees from Vertex Pharmaceuticals and grants from Christiane Herzog Foundation, during the conduct of the study. Conflict of interest: C. Joachim has nothing to disclose. Conflict of interest: I. Kirsch has nothing to disclose. Conflict of interest: T. Uselmann has nothing to disclose. Conflict of interest: Y. Yu has nothing to disclose. Conflict of interest: N. Alfeis has nothing to disclose. Conflict of interest: C. Berger has nothing to disclose. Conflict of interest: R. Minso has nothing to disclose. Conflict of interest: I. Rudolf has nothing to disclose. Conflict of interest: C. Stolpe has nothing to disclose. Conflict of interest: X. Bovermann has nothing to disclose. Conflict of interest: L. Liboschik has nothing to disclose. Conflict of interest: A. Steinmetz has nothing to disclose. Conflict of interest: D. Tennhardt has nothing to disclose. Conflict of interest: F. Dörfler has nothing to disclose. Conflict of interest: J. Röhmel has nothing to disclose. Conflict of interest: K. Unorji-Frank has nothing to disclose. Conflict of interest: C. Rückes-Nilges has nothing to disclose. Conflict of interest: B. von Stoutz has nothing to disclose. Conflict of interest: L. Naehrlich reports that he has received institutional fees for site participation in clinical trials from Vertex Pharmaceuticals. Conflict of interest: M.V. Kopp has nothing to disclose. Conflict of interest: A-M. Dittrich has nothing to disclose. Conflict of interest: O. Sommerburg has nothing to disclose. Conflict of interest: M.A. Mall reports grants from the German Federal Ministry of Education and Research and the Einstein Foundation Berlin during the conduct of the study; advisory board, consultancy, lecture and clinical trial fees from Boehringer Ingelheim, advisory board and consultancy fees from Arrowhead Pharmaceuticals, advisory board, consultancy, lecture and clinical trial fees from Vertex Pharmaceuticals, advisory board and consultancy fees from Santhera, consultancy fees from Galapagos and Sterna Biologicals, advisory board and consultancy fees from Enterprise Therapeutics, and consultancy fees from Antabio, outside the submitted work.
Figures
References
LinkOut - more resources
Full Text Sources
