TGFβ1 and TGFβ2 proteins in corneas with and without stromal fibrosis: Delayed regeneration of apical epithelial growth factor barrier and the epithelial basement membrane in corneas with stromal fibrosis
- PMID: 33263285
- PMCID: PMC7856119
- DOI: 10.1016/j.exer.2020.108325
TGFβ1 and TGFβ2 proteins in corneas with and without stromal fibrosis: Delayed regeneration of apical epithelial growth factor barrier and the epithelial basement membrane in corneas with stromal fibrosis
Abstract
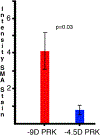
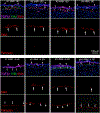
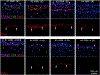
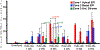
The purpose of this study was to investigate the expression and localization of transforming growth factor (TGF) β1 and TGFβ2 in rabbit corneas that healed with and without stromal fibrosis, and to further study defective perlecan incorporation in the epithelial basement membrane (EBM) in corneas with scarring fibrosis. A total of 120 female rabbits had no surgery, -4.5D PRK, or -9D PRK. Immunohistochemistry (IHC) was performed at time points from unwounded to eight weeks after surgery, with four corneas at each time point in each group. Multiplex IHC was performed for TGFβ1 or TGFβ2, with Image-J quantitation, and keratocan, vimentin, alpha-smooth muscle actin (SMA), perlecan, laminin-alpha 5, nidogen-1 or CD11b. Corneas at the four-week peak for myofibroblast and fibrosis development were evaluated using Imaris 3D analysis. Delayed regeneration of both an apical epithelial growth factor barrier and EBM barrier function, including defective EBM perlecan incorporation, was greater in high injury -9D PRK corneas compared to -4.5D PRK corneas without fibrosis. Defective apical epithelial growth factor barrier and EBM allowed epithelial and tear TGFβ1 and tear TGFβ2 to enter the corneal stroma to drive myofibroblast generation in the anterior stroma from vimentin-positive corneal fibroblasts, and likely fibrocytes. Vimentin-positive cells and unidentified vimentin-negative, CD11b-negative cells also produce TGFβ1 and/or TGFβ2 in the stroma in some corneas. TGFβ1 and TGFβ2 were at higher levels in the anterior stroma in the weeks preceding myofibroblast development in the -9D group. All -9D corneas (beginning two to three weeks after surgery), and four -4.5D PRK corneas developed significant SMA + myofibroblasts and stromal fibrosis. Both the apical epithelial growth factor barrier and/or EBM barrier functions tended to regenerate weeks earlier in -4.5D PRK corneas without fibrosis, compared to -4.5D or -9D PRK corneas with fibrosis. SMA-positive myofibroblasts were markedly reduced in most corneas by eight weeks after surgery. The apical epithelial growth factor barrier and EBM barrier limit TGFβ1 and TGFβ2 entry into the corneal stroma to modulate corneal fibroblast and myofibroblast development associated with scarring stromal fibrosis. Delayed regeneration of these barriers in corneas with more severe injuries promotes myofibroblast development, prolongs myofibroblast viability and triggers stromal scarring fibrosis.
Keywords: Corneal fibroblasts; Corneal scarring; Epithelial barrier function; Epithelial basement membrane; Fibrocytes; Fibrosis; Macrophages; Monocytes; Myofibroblasts; Perlecan; Secretory vesicles; Transforming growth factor beta-1; Transforming growth factor beta-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Proprietary interest statement
None of the authors have any commercial or proprietary interest in this study.
Figures






References
-
- Annes JP, Rifkin DB, Munger JS, 2002. The integrin αVβ6 binds and activates latent TGFβ3. FEBS Lett 511, 65–68. - PubMed
-
- Azar DT, Hahn TW, Jain S, Yeh YC, Stetler-Stevensen WG, 1996. Matrix metalloproteinases are expressed during wound healing after excimer laser keratectomy. Cornea 15, 18–24. - PubMed
-
- Ban Y, Dota A, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Mochida C, Kinoshita S, 2003. Tight junction-related protein expression and distribution in human corneal epithelium. Exp. Eye Res 76, 663–669. - PubMed
-
- Brown JC, Sasaki T, Göhring W, Yamada Y, Timpl R, 1997. The C-terminal domain V of perlecan promotes beta1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem 250, 39–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

